More than 660 million people lack access to clean drinking water. This intensifying problem due to growing population and shrinking water tables is driving innovation in drinking water disinfection systems, and the momentum is increasing as improvements in the performance of Ultraviolet C (UVC) light emitting diodes (LED) are matching the requirements of point-of-use (POU) systems.
UVC LEDs provide designers with a more effective germicidal light source; more design flexibility, which leads to more optimized systems; and reduced cost of ownership for the end user. Powerful UVC LEDs are figuring prominently into designs for everything from home and office appliances to commercial systems for water purification.
Access to the right germicidal wavelengths
In UV disinfection, light in the range of 250 to 280 nanometers (nm) disrupts the DNA of microorganisms, rendering them unable to reproduce. The action spectrum for bacteria is commonly reported as 265 to 275 nm peak wavelengths, although wavelength susceptibility may vary among a large number of bacterial and viral strains.
Traditionally, POU system designers have relied on low-pressure mercury arc lamps, which emit a discrete wavelength at 253.7 nm, to access this germicidal range. These lamps provide sufficient power for the systems and have become the industry standard.
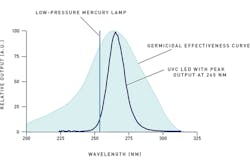
However, UVC LEDs are emerging as an alternative light source because of their design benefits over lamps. UVC LEDs are solid-state devices with a continuous emission spectrum across a specified range. The peak wavelength of the emission may be tailored to germicidal wavelengths with new technology in crystal growth for wide band gap semiconductors such as LEDs (see Figure 1).
Figure 1 shows that the low-pressure mercury lamp emission line intersects the germicidal effectiveness curve below the peak absorption. Although this is not the optimum germicidal wavelength, sufficient emission for DNA inactivation exists. The emission of the UVC LED delivers more overlap of the most critical wavelengths for disinfection, making it a more efficient light source for these systems.
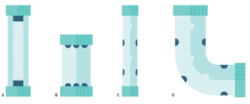
Figure 2. A. Flow cell with center-mounted mercury lamp. B. Flow cell with UVC LEDs mounted in end caps. C. Flow cell with UVC LEDs positioned inside the tube. D. Irregular-shaped flow cell with UVC LEDs mounted inside. In practice, LEDs would be separated from water with a leak-proof UVC transparent housing.
Design flexibility for flow cell size and shape
Optimum design of a water purification flow cell — the so-called UV Reactor — ensures UVC light is delivered in the most efficient and effective manner by maximizing overall UV dosage and delivering uniform light distribution so that no target microbes escape exposure. To achieve this, system designers consider flow cell size and shape, water flow rate in the cell, and the system’s UV power.
With traditional disinfection systems, the reactor design must adapt to the shape of a mercury bulb, which is a long, cylindrical tube (Figure 2A). This shape dictates the footprint of the UV unit in the water system, limiting the system designer’s options for overall size and configuration of the reactor. Conversely, UVC LEDs are a point light source in a durable, compact package, which creates an opportunity for maximum design freedom regarding the reactor footprint (see Figure 2B, C and D). The small size of the UV source enables smaller POU disinfection systems and the flexibility to address the maximum fluence rate and field uniformity to produce ever more efficient reactors.
Figure 3. Sketches of Reactor 1 and Reactor 2 concepts
Additionally, in applications where space is at a premium or increased efficiency is needed, system designers can optimize the field of uniformity by leveraging reactor designs with highly reflective inner surfaces. With this design, photons experiencing multiple internal reflections are recycled to contribute to the cumulative disinfection dosage. The most common material in traditional UV reactors has been stainless steel, which only has 28 to 33 percent reflectance of UV light. Alternatives include expanded polytetrafluorethylen (PTFE), which provides more than 90 percent reflectance in the UVC range or almost three times more effectiveness than traditional reactors.
Improving efficacy of reactor design
The primary concern when designing UV reactors is sufficient disinfection to meet well-established dosage guidelines for bacteria, viruses and protozoa from standards committees, such as NSF International. Many designers are simply targeting an average dosage established by these guidelines to create an effective system. Generally, this requires an understanding of the uniformity of UV fluence as well as maximizing UV power and exposure time of microbes to UV light to meet a required dosage.
Overall, a designer first considers germicidal power in the reactor as disinfection dosage is calculated as UV intensity multiplied by exposure time. The second parameter, exposure time from the dosage equation, can require more interpretation, modeling and design. Flow cell designers often refer to the term as "residence time," which is the average time water spends in the reactor. The longer the residence time, the more microbes have a chance to be irradiated by germicidal UV light.
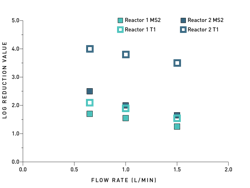
Figure 4. Comparison of Reactor 1 and Reactor 2 for flow cell for MS2 and T1 reductions
The example below compares two reactors for water disinfection with identical germicidal UV power but varied reactor design. Both systems process the same amount of water, have the same overall dimensions and have the same UVC LED arrangements. While these variables remained fixed, the designs were altered to maximize the residence time by shifting the position of the water inlet and outlet. For both reactors, the maximum flow rate is 1.5 liters per minute. Reactor 1 has a residence time of 3.09 seconds, while Reactor 2 has a residence time of 3.76 seconds.
A series of tests evaluated the efficacy of the reactors at three flow rates with a UV transmittance of 90 percent and a single power input of 100 percent. The influent was seeded with MS2 and T1 coliphage, which are common challenge organisms used in water system designs. The tests measured the concentrations of the challenge microbes present in the influent and effluent to determine the log reduction. Figure 4 shows the difference in log reduction at varied flow rates for microbes MS2 and T1 in each reactor.
Simply put, the longer residence time of Reactor 2 at a given flow rate results in a more effective inactivation of the MS2 and T1.
Reducing cost of ownership
Reducing cost of ownership offers a key product differentiator in POU systems. Traditional mercury lamps require a long warm up time, anywhere from 50 seconds to 10 minutes, to reach the required germicidal intensity. In addition, frequent on/off cycles can diminish lifetime by 50 percent or more. Consequently, mercury lamps in these applications are often kept on all day, increasing the frequency of lamp replacement and power consumption.
By contrast, the instant on-off capability of UVC LEDs enables on-demand disinfection, which reduces power consumption. Additionally, the frequent on/off cycles do not diminish LED lifetime, helping lower operating and maintenance costs. Finally, no concern for disposal of hazardous mercury comes with LEDs, addressing another element of lifecycle cost. Table 1 provides a five-year cost analysis for the mercury lamp-based and UVC LED-based systems.
High performance UVC LEDs are allowing manufacturers to migrate from mercury lamps to solid-state lighting solutions. The higher efficiency in the germicidal wavelengths and compact footprint of UVC LEDs enable more effective disinfection systems for safe drinking water. With demonstrated effectiveness in water and air disinfection for more than 99.99 percent of germicidal efficacies, little question remains that these light sources are a viable alternative to traditional methods and will continue to trend in innovative new designs. With lifetime cost savings of 57 percent over five years, consumers are sure to adopt it.
Rajul Randive is the director of applications engineering at Crystal IS and is responsible for designing, building and testing prototype applications that use UVC LEDs. He is a frequent industry author and a voting member on the ASHRAE Committee for "Guidelines for the application of ultraviolet germicidal devices to control the transmission of airborne pathogens." He also holds a patent for a high throughput vaporizer (Patent #6789789). Randive has an MBA from the State University of New York at Albany, a Ph.D. in organic chemistry and an MS in chemistry from Clarkson University in Potsdam, New York.