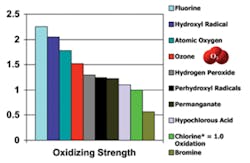
Within the microbial world protozoa, such as crypto, are some of the most resistant to all types of disinfectants. The reason for this resistance is due to its hard outer protective shell, which must be broken through prior to the microbe being inactivated. Crypto can cause a variety of ailments, including abdominal cramping, diarrhea, fever and nausea that can last as long as a month, according to the Centers for Disease Control and Prevention (CDC).
Typical disinfectants used to ward off cryptosporidium for most water treatment applications are chlorine (liquid state), chloramines, chlorine-dioxide (gaseous state) and ozone. However, their ability to perform this inactivation duty should not be regarded equal, as each sanitizer requires a specific level of concentration and contact time to take effect.
Ct Values
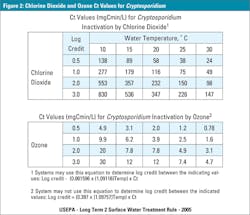
To better determine the specific amount of the disinfectant required to inactivate or destroy a microbe, the Environmental Protection Agency (EPA) has determined precise Ct Values. These Ct Values are the product of the disinfectant’s concentration (C, expressed in mg/L) and the contact time (t, expressed in minutes). These Ct Values are calculated specifically to the percentage of microbial kill or better known as the log reduction, e.g. 1-Log = 90.0 percent, 2-Log = 99.0 percent or 3-Log = 99.9 percent inactivation of the particular microbe. Figure 2 provides EPA Ct Values for cryptosporidium based on the desired log reductions and temperature for both chlorine dioxide and ozone respectively. According to the EPA, chlorine dioxide would require a Ct of 226, which would correlate to 226 mg/L, at one minute of contact time, at 25° C to achieve a 3-Log reduction or 99.9 percent inactivation. Although, ozone would only require a Ct of 7.4, correlating to 7.4 mg/L, to achieve the same 99.9 percent inactivation with the same parameters as chlorine dioxide (EPA, LT2ESWTR, 2005).
Note: Because Ct is a product of concentration and time both can be manipulated, as long as the given Ct Value is obtained for the desired log reduction (e.g. Ozone Ct of 7.4 can be achieved with a concentration 3.7mg/L for two minutes of time).
Cryptosporidium outbreaks in our public drinking waters and recreational swimming pools are becoming more and more of an evident issue. Unfortunately, forms of chlorine sanitation are not often the best solution, especially for high organic and inorganic contaminant levels, as they will create chlorine oxidation by-products, such as trihalomethanes (THM) and chloramine derivatives. These by-products are the typical cause of (what most associate as being over chlorinated) the chlorine smell in drinking or pool waters, and are the cause of itchy, smelly skin and burning eyes in pool water. Although with a properly sized system, ozone can be used as the primary sanitizing and oxidizing agent, oxidizing the contaminants completely. Using ozone in this manner would then allow chlorine to be used as the secondary residual sanitizer to satisfy regulatory requirements, without the production of chloramines and chlorine’s side effects.
UV water treatment
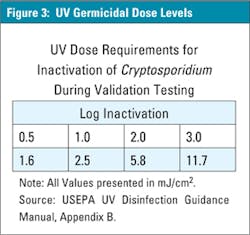
Another typical disinfectant used to battle microbes, including cryptosporidium, is the UV germicidal sterilizer. A germicidal sterilizer produces UV light at 254-nanometer (nm) wavelength and has been proven to inactivate microbes not by their destruction, but rather by scrambling the DNA and RNA, rendering the microbe sterile (WHO, Water Treatment and Pathogen Control, LeChevallier and Au, 2004). Water flows through the UV sterilizer and passes the UV light, which is protected by a quartz sleeve, keeping the water from coming in contact with the lamp. Like other oxidizers, such as ozone, UV systems too have specific dose rates that can be used to determine an effective log reduction. Figure 3 indicates the dose rate of UV required to inactivate cryptosporidium.
This specific dose rate can be found by the product of the UV light intensity (where, I = Intensity, mJ/cm2) and the contact time (where t = Exposure time in seconds), (EPA, Alternative Disinfectants and Guidance Manual, 1999).
However, UV is often hampered by poor water quality caused from particulate, tannins, color or even scaling from hard water, preventing the light from having a clear line-of-sight to the pathogen. Without this line-of-sight the microbe can be allowed to flow through the germicidal sterilizer undetected and with the ability to still reproduce.
A true benefit of ozone water treatment is that it does not just sterilize cryptosporidium and other microbes, but it effectively kills the microbes in addition to providing oxidation of other contaminants that may be in the water, such as color, tannins, metals, etc. Therefore, regardless of the line-of-sight, ozone holds no prisoners.
Water treatment professionals are leveraging ozone’s capabilities by providing their customers with the strongest oxidizer on the planet, while eliminating the most harmful microbes that continue to infiltrate our lives on a daily basis. Ozone can provide a great resolve for many water quality issues. Find out how ozone can help you.
<