On-Site Disinfection for Beverage Industry Sanitation
In beverage bottling and canning plants, Clean-in-Place (CIP) is used to clean various product lines such as syrup and water lines for a variety of reasons including flavor carryover prevention and microbial control. CIP also eliminates organic residues like precipitated proteins, carbohydrates, fats, minerals and other contaminants that harbor bacteria and may lead to microbial induced corrosion (MIC).
The term CIP indicates that the cleaning process is largely done in-situ, where the vast majority of piping, tanks, pumps and other components are left “in-place” and not disassembled for cleaning. Particularly in industries that require stringent hygiene like the beverage, brewing, dairy and processed foods industries, CIP offers a rapid labor saving and more repeatable method to maintain scrupulously clean systems.
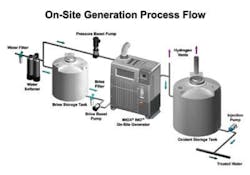
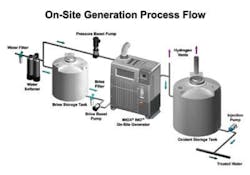
Figure 1. On-Site Generation Process Flow -- Illustration of some of the basic components of an OSG including (a) water softener, (b) brine tank, (c) brine pump, (d) water heater/chiller, (e) electrolytic cell and cell controller, (f) oxidant tank,(g) metering pump, and (i) hydrogen vents.
Although the CIP process is a relatively short-cycle process, it can still be one of the more time-consuming aspects of plant operation and maintenance. Time lost to CIP is also valuable lost production time. In addition, traditional CIP methods heat the cleaning solution to improve effectiveness. As a result, many producers are constantly looking for ways to reduce their CIP cycle times without sacrificing safety, complexity or burdensome additional cost.
One option is the use of On-Site Generation (OSG) of oxidant electrolytically using a generator, such as MIOX’s mixed oxidant technology, which has been used for over 15 years in 30 countries and is ideal for CIP beverage processing. The mixed oxidant CIP cleaning solution used in the Miox system is an environmentally benign and single-component solution that replaces 4- and 5-step CIP processes with a 3-step cold-CIP process consisting of rinse, treatment, and final rinse. The company’s non-thermal technology reduces energy consumption, and the rapid cleaning cycle significantly improves beverage facility production rates. The process reduces costs by generating sanitizer on site, on demand, increasing efficiency and increasing valuable beverage production time.
On-Site Generation
Based on decades-old scientific principles, on-site disinfection generators apply electricity to a solution of salt and water, which produces chlorine and other oxidant species. OSGs have a number of industrial applications - including providing regulatory approved disinfectants in the beverage process, power plants, and swimming pools – and are used to treat municipal water to US EPA drinking water standards.
One example of a mixed oxidant system is the MIOX® CIP. The non-thermal technology reduces energy consumption, and the rapid cleaning cycle -- rinse, treat, final rinse – saves time and money by increasing plant production.
OSGs produce chlorine when a solution of sodium chloride is passed through an electrolytic cell and electricity is added. Incoming water goes through an ion exchange water softener to remove calcium. Softened water feeds the electrolytic cell while a soft water sidestream fills a brine tank, which generates a concentrated salt solution. The near-saturated brine is then injected into the softened water stream entering the electrolytic cell.
When the dilute salt solution is inside the electrochemical cell, current passes through the cell producing a strong oxidant solution. After exiting the electrolytic cell, the oxidant solution is stored in an oxidant tank.
The electrolytic cell is fundamental to the OSG. Electrolytic cells consist of two electrodes, the anode and cathode, designed so that both make contact with the mixed water and brine solution. A voltage is applied to the cell so that current flows through the cell, causing chemical reactions to take place at the surfaces of both electrodes, producing the disinfectants. Oxidation reactions are carried out at the anode where two chloride ions (Cl-) are stripped of one electron each to produce chlorine which is dissolved in the solution.
The geometric, hydraulic and power configuration of the cell makes it possible to produce oxidants other than chlorine that can provide enhanced removal of microbiological contaminants. The strong chlor-oxygen based solution is delivered to the oxidant storage tank at a pH of 9, thereby reducing corrosion effects in CIP piping.
Chlorine production is balanced by the reduction reactions that occur at the cathode where water (H2O) is converted into hydroxide ions (OH-) and hydrogen gas (H2).
Hydrogen gas in the form of bubbles is produced during electrolysis at the cathode. Passive and active ventilation systems remove the gas from the OSG and piping before it can enter the oxidant storage tanks, improving system safety.
Beverage Processing Benefits
On-site generation offers significant sanitation benefits for beverage processing, including increased beverage production, chemical cost savings, improved safety, more effective sanitation, and greener applications.
Because there is no need to continuously purchase chlorine chemicals, OSGs typically produce chlorine at a much lower cost than traditional delivery methods as the only consumables are salt and electricity used to generate the chemical. In fact, many beverage plants will already have food-grade salt available, simplifying procurement of the only “chemical” required for the generator. Decreased transportation and safety-related costs, and lower insurance premiums offer additional savings.
Although OSG systems can present a larger up-front capital equipment cost, most beverage processing plants realize a return on their investment in a short period of time.
Produced on-site, on demand, the mixed oxidant solution is an inherently safer beverage CIP disinfectant. The solution produced has a relatively low concentration with moderate pH, unlike other “quick CIP” chemicals such as peracetic acid.
Since mixed oxidants are effective at eliminating biofilm, bacterial contamination is reduced or eliminated and disinfectant requirements are reduced; and a more durable disinfectant residual safely prevents recontamination. Taste and odor carry-over are eliminated when switching from one beverage to the next, plus the solution is easily rinsed from the system, eliminating residual “chlorine” taste and odor quickly.
With fewer organics in beverage distribution piping, fewer disinfection by-products are formed and microbial-induced corrosion (MIC) is reduced. Compatibility of the solution with existing materials of construction (304 and 316 stainless steel, etc) are excellent and below industry standards for corrosion.