By C.F. “Chubb” Michaud
• Proper control of water chemistry in boiler and heat recovery steam generation (HRSG) operations is crucial to an effective, cost efficient high purity system.
The traditional method currently used to produce “high purity” (i.e., >10 megohm) water for industrial processing is to utilize a two bed demineralizer or a reverse osmosis (RO) system as a primary followed by a mixed bed (MB) demineralizer polisher. If the raw water exceeds about 200 ppm, usually RO is the more
economical choice. For smaller systems (those under 50 gpm), portable exchange mixed bed deionization (PEDI) service has its advantages – there’s no complicated mixed bed to separate and regenerate on-site. With PEDI, the entire responsibility for maintenance and quality rests elsewhere. But what if you’re in a remote area or just want to do it in-house for control? There is an option for producing quality 10 meg water without using mixed beds.
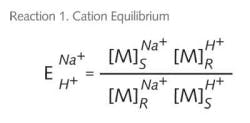
If you look at the equilibrium chemistry between sodium and hydrogen for a strong acid cation (SAC) exchanger – we’ll use NaCl as the load for this example with the resin in the hydrogen form – we see that ions in solution are selectively picked up on the resin in exchange for ions on the resin.
As illustrated in Reaction 1, the driving forces that determine equilibrium (E) or the completeness of this reaction are determined by the concentration [M] of the sodium ions in solution (S) and the hydrogen ions on
the resin (R). Competing against the complete conversion are the concentrations of the sodium ions [M] on the resin (R) and the hydrogen ions in solution (S). We write the ion exchange ion exchange reaction as such (see Reaction 2).
With the salt converted to acid by exchanging the Na+ ion for H+ ion, we now enter the strong base anion (SBA) bed with the reaction in Reaction 3. The SBA resin exchanges the Cl- ion for OH- ion and produces DI water.
The Real World vs Theory
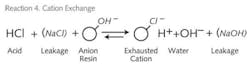
We can picture that, as the NaCl solution enters the freshly regenerated resin bed, Na+ is picked up by the resin and H+ is released into the solution. As the solution travels further down the column, the solution concentration of the Na+ ion decreases and the H+ ion increases. From our equilibrium Reaction 1, we see this will slow things down. In addition, the Na+ ion on the resin is increasing and the H+ on the resin is decreasing. Again, driving forces for completing the reaction are diminished. In addition, as the concentration of the H+ increases while moving down the bed it will equilibrate with any residual Na+ ion still on the bed from the prior regeneration and pull Na+ back into the water phase. This is a very mild regeneration, but it results in the presence of Na+ in the effluent and is referred to as “leakage.” Leakage is assumed to be Na+ since it’s the least tightly held ion in the feed water. One should also note that whenever an ion exchange reaction moves the pH away from pH 7, it will retard the reaction. So the cation exchange reaction is fighting the equilibrium and pH shift right from the start. A more appropriate representation of what happens in two bed DI systems is shown in Reactions 4 and 5.
Leakage in a typical 7-10 lb HCl regenerated cation exchanger bed will be in the range of a half of 1% of total cations. If we have a 250 ppm of total cation in the feed, we can expect Na+ leakage of 1.25 ppm. While the acid produced in the SAC is totally neutralized and rendered as HOH, the NaCl leakage converts to NaOH. Depending on how good a job one has done on the SAC regeneration, the two bed effluent pH will reflect 1-1.5 ppm of NaOH – around a pH of 9.4 to 9.6. Since the conductivity of NaOH is about 2.5 times that of NaCl, the readings will look more like 3-4 ppm of ionic leakage. Quality-wise, 3-4 ppm (of NaCl) reads as 100-150K ohms. If you use more acid for regeneration, this reading may climb to 250-400K, but you’ll never see 1 megohm water [1 megohm = 1 microsiemens (µS)] with conventional two bed chemistry.
Adding a layer of complexity but still keeping with simplicity of two bed is “counter-flow” regeneration, where the fresh regenerant chemical is run counter to the service flow (assume service is down-flow). To be successful, the beds must be held in place during regeneration, which is usually up-flow. This is to preserve the polishing zone at the bottom of the bed. The blocking flow of water is the added layer of equipment. A regenerant take-off lateral positioned just above the resin bed serves as the outlet for the acid regenerant and blocking water. Counter-flow systems are backwashed infrequently to keep the polishing zone at the bottom of the bed intact. The advantage of counter-flow is lower leakage. According to the literature, leakage is only about 10-20% of conventional regeneration. We could expect Na+ leakages of 0.1-0.25 ppm and a quality reading of 700,000-1,000,000 ohms.Another variation suggested on raw water feed streams that may require frequent backwashing due to high suspended solids in the feed or lack of pre-filtration is the “split-flow). Here, there’s a mid-lateral in the mid-section of the resin bed. Backwash is done on the upper half only by introducing the b/w flow to the mid-lateral and taking it out the top. Acid (or caustic) is introduced both up-flow and down-flow acting as the blocking flow. Results are similar to the pure counter-flow and this method produces less waste water. Conventional, counter-flow and split-flow systems are shown in Figure 1.
But we still have Na+ leakage. This is where the mixed bed comes in. A mixed bed (MB) polisher will easily boost the resistivity of a well run two bed all the way to 18 megohm. But what if you don’t need THAT level of quality and want to keep things simpler (i.e., the K.I.S.S. principle).
Two Bed Polishing without Mixed Bed
We can easily polish up the Na+ leakage from the two bed with the addition of another SAC vessel. Three bed operation using SAC/SBA/SAC is becoming more popular for operations needing 1-10 meg water but are afraid of operating a MB. Conventional flow three beds will produce 1-3 meg water while a counter-flow or split-flow three bed can top 10 meg. Na+ leakage will be reduced by 90+% with the additional SAC bed. Reaction 6 assumes the effluent from the SBA contains only a little Na+ leakage as NaOH.
While three bed water will produce great quality, it won’t be the same as mixed bed quality… yet. Na+ leakage from the primary cation will convert to NaOH in the SBA. This will cause silica leakage by producing a mild regenerant that will cause silica to slough off of the partially exhausted anion. The better the cation regeneration, the lower the silica will be. If ultra-low silica is your reason for using a mixed bed, try using counter-flow acid on your cation. Counter-flow three bed units will produce mid-range mixed bed quality… including low silica.
Taking this to the next level, a five bed train consisting of SAC/SBA/SAC/SBA/SAC will produce 18 meg with <5 ppb sodium and <5 ppb silica. This has been demonstrated by the Purolite Company using packed bed technology to facilitate the counter-flow technology.
About the Author: Chubb Michaud is a 30-year veteran of the industrial water industry and holds three U.S. patents on methods of minimizing regenerant waste. A past chair of the Industrial Section and Ion Exchange Committee of the Water Quality Association, he’s currently technical director of Systematix Company of Buena Park, CA. Contact: www.systematixusa.com