Examining Antiscalants for Calcium Fluoride Deposition Control at RO Plants
By Suresh Patel
In the environment fluorides occur both naturally (rock weathering, volcanic emissions) as well as due to human activities (phosphate rock mining, steel industry, drinking water fluoridation). In natural waters, concentration of fluoride is low, ranging up to 5 ppm; however certain wastewaters from steel or mining plants have increased fluoride content of up to 80 ppm.
In order to comply with environmental regulations, as well as to reduce costs, steel and mining process waters are often recycled in reverse osmosis plants operating at high recovery (70-80%). In these conditions brine waters that have highly concentrated fluoride and calcium content exceed the supersaturation of calcium fluoride and form a precipitate due to low calcium fluoride solubility in water (16 ppm at 20°C).
This article examines laboratory studies that were carried out to investigate additives that can control calcium fluoride precipitation in waters with high calcium fluoride saturation indices of up to 300.
Analysis of Waters Containing High Fluoride Content
Typical water that is found in steel industry plants contains fluoride levels of up to 80 mg/l. The studies focused on wastewaters from two steel mill plants in Asia. The waters are recycled in reverse osmosis plants at recoveries of around 70-80%.
Analysis on these two waters was carried out and calcium fluoride saturation indices were calculated using the following equation:
Saturation level greater than 1 indicates that the solubility of calcium fluoride is exceeded and its precipitation is likely to take place. Also, the larger the saturation index value, the greater the potential of calcium fluoride precipitation.
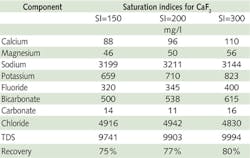
Three water chemistries mimicked the three saturation indices for calcium fluoride at 150, 200 and 300 that are likely to be found in the RO concentrate stream at corresponding recoveries of 75%, 77% and 80%. These water chemistries are shown in Table 1.
All three waters would give rise to severe precipitation of calcium fluoride and hence would be acceptable to evaluate additives for their efficacy.
Experimental
Three additives were tested in this work, Antiscalant A is based on modified polymaleic acid chemistry, Antiscalant B is based on polycarboxylic acid chemistry and Antiscalant C is based on phosphonate chemistry.
Calcium Fluoride Static Test
The purpose of this study was to identify if calcium fluoride precipitation can be controlled in RO concentrate with high calcium fluoride saturation indices. A laboratory method was developed based on three synthetically prepared waters with high fluoride and calcium concentrations (Table 1).
Separate cation and anion solutions were made up. The solutions were mixed in equal proportions to obtain the desired calcium fluoride saturation levels of 150, 200 and 300 and additives A, B or C were added to the required concentration.
The samples were then shaken and left standing at room temperature for an hour, or 30 minutes for tests carried out at SI of 300 (due to scaling severity). Presence of turbidity was noted, and the samples where filtered and titrated for calcium level left in solution.
The precipitate formed under blank conditions (with no additives present) was collected, dried and physical analysis carried out. X-Ray diffraction analysis indicated that calcium fluoride crystals had formed and scanning electron microscopy indicated the size of the crystals ranged from 0.5 to 2 um.
Calcium Fluoride Dynamic Membrane Test
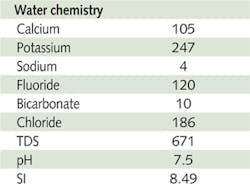
To further evaluate the efficacy of additives, a dynamic membrane test was developed. A reverse osmosis membrane with active surface area of 91cm2 (7x13 cm) was used in the test. The membrane was placed in a Millipore Minitan Filtration cell. Calcium fluoride test water was prepared (water chemistry in Table 2) and pumped through the RO membrane under the pressure of 10 psi.
Change in permeate flux was monitored over four hours in test solution with addition of 1 ppm of antiscalant which was the compared with a blank (no additive) and for reference a sodium chloride ( 650 ppm NaCl).
Results and Discussion Calcium Fluoride Static Test
Three different Antiscalants A, B and C were tested in the laboratory. The method established that in blank conditions (without additives) calcium fluoride precipitation in waters with high calcium fluoride saturation index (SI) of 150, 200 and 300 is rapid (3-8 minutes).
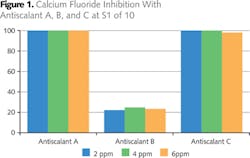
Figure 1 shows the results of activity of the antiscalants tested in water with a calcium fluoride saturation index of 150. These results show that Antiscalant A and Antiscalant C effectively controlled precipitation of calcium fluoride from dose level of 2 to 6 ppm. Antiscalant B was not effective, even when the dose level was increased to 6 ppm, therefore it was not considered for further testing.
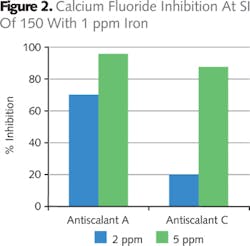
In order to differentiate between the activity of Antiscalant A and Antiscalant C, 1 mg/l iron was added to the water since many mine waters contain heavy metals. Figure 2 shows that Antiscalant A was much more effective at 2 ppm and 5 ppm dose levels compared to Antiscalant C.
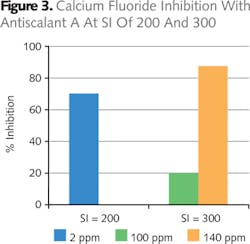
Based on these findings, Antiscalant A was then tested in more severe waters (higher SI of 200 and 300). At a calcium fluoride saturation index of 300, water proved to be extremely severe, hence a higher dose level of antiscalant to control the precipitation (Figure 3).
Calcium fluoride dynamic membrane test
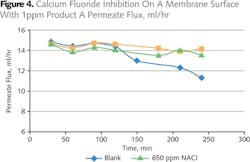
Antiscalant A was selected for further investigation in the dynamic membrane test. The test water in Table 2 was used with 1 ppm Antiscalant A. Figure 4 showed that no significant permeate flux was lost during the four hour duration of the test, while the flux under blank conditions (no additive present) started to decline rapidly after 120 minutes of the test. Permeate flow obtained during the reference test run with 650 ppm sodium chloride was consistent with the 1 ppm Antiscalant A test run over the four hour test period indicating no deposition taking place on the membrane surface.
Conclusions
Antiscalant A was the most effective in inhibiting the precipitation of calcium fluoride. In water with calcium fluoride saturation indices of 150 and 200, the dose levels needed were low, even when 1 ppm iron was introduced. It was also effective in severe water at calcium fluoride saturation index of 300, although the dose level had to be significantly increased in order to inhibit the precipitation. In presence of iron, it also had better tolerance than Antiscalant C.
Antiscalant C was also found to be effective however some of its activity was lost due to iron poisoning. Therefore its use in steel and mining industries where the wastewaters often contain heavy metals will be limited.
Antiscalant B had very little control of calcium fluoride and was found to be least effective in controlling the formation of calcium fluoride in this work.
About the Author: Dr Suresh Patel obtained his PhD at Loughborough University followed by Postdoctoral Research at Imperial College, London University. He is a Technology Manager in the Research and Development Laboratories of BWA Water Additives Business in Manchester. His main area of work is on antiscalants and corrosion inhibitors for water treatment and in particular antiscalants for thermal and membrane desalination. He has 6 patents and over 20 publications in water treatment.