Membrane Fundamentals, Part3: Effect of pH, Temperature Chemicals and Cleaning Procedures vs. Foulant Removal
This series of articles discusses cleaning of reverse osmosis (RO) systems. The first article, published in the January/February 2006 issue of Industrial WaterWorld, discussed cleaning criteria and normalization of RO systems. The second article, in the May/June issue, discussed design and operation of a clean-in-place (CIP) skid and its integration in an RO system. This third article discusses the importance of pH and temperature, cleaning chemicals, and cleaning procedures for effective removal of foulant from RO membranes.
Hot, cold, basic, acidic
The solubility of a salt or organic compound is temperature-dependent and is generally found to increase with higher temperatures. Therefore, increasing the temperature of the cleaning solution improves removal of foulant from a membrane surface.
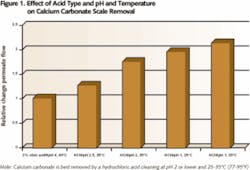
The solubility of many salts and organics also is affected by pH. For instance, calcium carbonate (CaCO3) scale starts to dissolve when the pH of a cleaning solution is lowered below 7. The minimum temperature of the cleaning solution shouldn’t be below 68°F (20°C) because of very slow chemical kinetics at low temperatures - and chemicals such as sodium lauryl sulfate may precipitate at low temperatures. The graphs in Figures 1 and 2 show the effect of temperature and pH on foulant removal. For instance, cleaning at pH 12 is effective to remove biofouling, while cleaning at pH 10 has little result. CaCO3 is best removed at pH 1-2 and at elevated temperatures (above 77°F/25°C).
Four important questions
Which pH and temperature should be applied for an RO system? - Prior to cleaning, it’s important to determine which elements are installed in the RO system. The type of membrane element and model determine cleaning pH and temperature limits. Each membrane manufacturer has its own guidelines for cleaning. For instance, some manufacturers recommend pH limits of 3 and 11 for cleaning, while others recommend pH limits of 1 and 13. In addition, different temperatures are recommended depending on the chosen pH value for cleaning.
Exceeding the membrane manufacturer’s guidelines can cause membrane damage, which can result in a salt passage increase. Therefore, it’s strongly recommended guidelines, which can be obtained from the manufacturer or master distributor, be followed.
How to determine which cleaning procedure should be applied? - Ideally, one knows exactly what’s present on the membrane prior to cleaning, which would allow the cleaning method to be tailored to the identified foulant(s). In practice, one may not know what kind of foulant is causing the performance decline of an RO system. There are some methods, though, that can be used to determine which cleaning procedure should be applied:
- a. First stage is an indication of colloidal or suspended solids fouling, biofouling and organic fouling
- b. Last stage is an indication of scaling, biofouling and organic fouling
- a. Well water - scaling and iron fouling
- b. Surface water - scaling, colloidal, suspended solids, organic and biofouling
- c. Municipally treated water - scaling, colloidal, organic and biofouling
The above methods help determine what kind of cleaning procedure is needed, such as alkaline, acid or both.
If a membrane is fouled, it’s typically a combination of different foulants, especially in the case of RO systems operating on pretreated surface water. Fortunately, certain cleaning procedures may remove different kind of foulants within a single procedure. An alkaline cleaning step is effective in removing organic fouling, biofouling and colloidal fouling. An acid cleaning is effective in removing calcium carbonate and iron oxide.
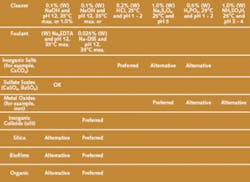
Table 1 contains information on specific foulants and recommended cleaning methods to remove them.
Which cleaning procedure first? - The majority of fouling problems observed in RO systems are typically organic fouling, biofouling and colloidal fouling. Scaling is rarely seen but, when it does occur, it’s typically due to a problem in the pretreatment, such as ion exchange softener problems, acid and/or antiscalant dose pump failure, or high recovery operations.
Typically, different types of foulants are present on the membrane surface that may require both acid and alkaline cleanings. The sequence of cleaning steps can be critical. Some membrane manufacturers and some membrane chemical manufacturers promote acid cleaning first, while others recommend alkaline first. The author recommends alkaline cleaning first, especially when biofouling, organic fouling and colloidal fouling are suspected. This is applicable for most RO systems operating on pretreated surface water or city water.
The reason for alkaline cleaning first is acid cleaning isn’t able to hydrolyze and solubilize the protective layer around microorganisms. Instead, acid cleaning “tightens” the biofilm, which can cause further performance decline of the membrane. An alkaline cleaning might not be able to restore the membrane performance decline caused by the acid cleaning. Therefore, alkaline cleaning is recommended as the first cleaning step.
What kind of water should be used? - RO permeate or deionized water should be used for preparation of the cleaning solution and for rinse-out of cleaning solution from the RO system.
Raw or municipally pretreated feed water should be avoided as its components may react with the cleaning solution: