In a Zenon Environmental presentation a few years ago, the words on a slide captured my attention as being both accurate in the moment and a predictor of the future. The words were: “Membranes are to water treatment what the microchip is to information technology.” The boom in membrane water treatment over the past few years, with relatively high growth rates in existing market segments and expansion into new market ones, I think, validates that prediction.
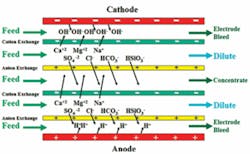
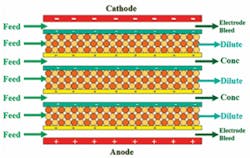
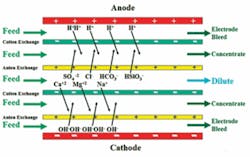
There are two general categories of membrane water treatment technologies. These are: