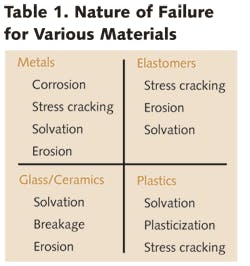
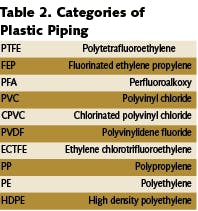
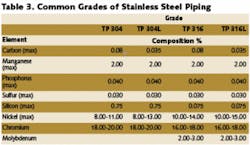
When considering materials of construction for water treatment systems, the following factors must be considered:
• Cost
• Durability
• Ease of fabrication
• Inertness
The last factor requires further explanation. Because treatment systems are being used on more diverse streams than ever before in the wastewater and chemical processing arena, they may encounter chemical and/or temperatures extremes that are capable of attacking piping, tanks, valves, etc. Also, the demand for high purity or ultrapure water for many applications has underscored the requirement that materials of construction be unaffected by this treated water which can act as an extremely aggressive solvent.
Choices in Composition
Not only must these materials not degrade treated water quality, but they also must be resistant to attack by aggressive solutions (the purified water itself, as well as cleaning and disinfecting chemicals), not to mention erosion by high water velocities. Black iron, cast iron and carbon steel materials have been virtually completely replaced by various plastics that are not only more chemically resistant, but provide benefits in weight, ease of fabrication and installation. For those applications requiring high temperature and pressure resistance beyond capabilities of plastics, new grades of stainless steel can fill the bill.
In wastewater and chemical processing applications, materials selection must be based on resistance to specific chemistry, temperature and/or pressure associated with the feed stream, and chemicals and conditions of cleaning/disinfecting operations. These generally fall into the categories of rigid PVC or polypropylene for low temperature and/or pressure applications and 316L stainless steel for high temperature and/or pressure applications. In those cases where the feed stream is high (>1,000 ppm) in chlorides or any other contaminant known to attack stainless steel, such exotic materials as titanium or Hastelloy may be required.
In those applications where raw water (municipally treated or untreated groundwater, surface water, etc.) is to be purified to meet a particular use requirement, construction material concerns are orientated toward preventing the aggressive purified water from attacking the materials and causing contamination.
When designing any treatment system, it’s often very useful to break it down into three components: pretreatment, primary treatment, and post treatment (storage and distribution).
Pretreatment
In this component, the water is treated with technologies such as filtration to protect the downstream primary treatment technologies from effects of waterborne contaminants on their performance. Pretreatment often involves particulate filtration, chlorine removal, pH adjustment or some other chemical addition step.
Plastics such as rigid polyvinyl chloride (PVC), high-density polyethylene (HDPE) and fiberglass reinforced polyester (FRP) materials are usually used in the pretreatment process. In those cases, where durability may be a concern, carbon steel may still be used.
Primary Treatment
In this component, the water attains its final quality while utilizing technologies such as membrane separations, ion exchange, electrodeionization (EDI), etc.
The materials of construction in this step must not only be inert to the finished water quality - not contaminate the treated water - but also be able to tolerate the chemistries, flows and pressures required for regenerating or backwashing the technologies, as well as cleaning and disinfection. Here, more than in any other step, a wide range of water qualities is encountered by the materials of construction. In terms of treatment technologies, softening would produce the least aggressive water, while mixed bed deionization or EDI produces the most aggressive water.
For the former, PVC, FRP and HDPE are perfectly acceptable, whereas for the latter, polypropylene or polyvinylidine fluoride (PVDF) constitute the most commonly used housing and piping materials, although there are other equally inert materials which are extremely effective.
Post Treatment
Once the water has been purified to its final quality, it’s extremely important that the storage and distribution system be designed to have as little effect on the treated water quality as possible. Ultrapure water, such as that produced by mixed bed ion exchange or EDI, will attack all of the known materials of construction, albeit very slowly. The point is, when water is purified to meet some extremely stringent specification, any contaminant, no matter how small, is deleterious. A similar effect can be seen with strong disinfectants such as ozone. It’s such a powerful oxidizing agent that it also attacks all materials of construction.
In ultrapure applications, where water quality cannot be compromised, the distribution system will include various “polishing” technologies such as exchange tanks of DI resin, ultrafiltration (or RO) membranes and 185 nm UV units to restore the quality of the purified water.
Biofilms
In virtually all water treatment systems, bacteria represent a significant problem. Because bacteria are prevalent everywhere and are viable (capable of reproducing) in almost every environment, the challenge isn’t how to eliminate them - this is virtually impossible - but how to keep them under control.
Most waterborne bacteria produce biofilms, a glycocalyx matrix, which adhere to any and all surfaces. Biofilms not only protect bacteria from disinfectants, but they also tend to capture and release other contaminants.
Although there’s no current material used in water treatment systems to which biofilms cannot attach, there are some general “rules of thumb” that will help minimize biofilm formation. Smooth surfaces provide less opportunity for the biofilm to adhere. The inside surface of a PVC pipe may appear smooth; however, to a bacterium it’s as cratered as the surface of the moon. The fluorinated polymers such as PVDF and PTFE have dense (smooth) surfaces that inhibit biofilm formation.
There’s considerable development under way in the area of “bacteriostatic” surfaces generally containing some form of silver, which appear promising in this area.
System design and operation will also aid in diminishing biofilm formation. Maintaining a minimum velocity through the pipe of 5 feet per second helps, although too high a velocity (>10 ft/sec) can result in erosion of the pipe material.
Conclusion
The removal of contaminants from a wastewater stream, the fractionation and recovery of specific solute in a chemical processing application, or the purification of raw water for a downstream use requirement present two challenges:
• The selection of the optimum treatment technologies;
• The selection of materials of construction to ensure that the treatment technologies will operate successfully without degradation and without compromising the quality of the treated water.
Both must be met to create the optimum design.
About the Author: A registered professional engineer and member of Industrial WaterWorld’s Editorial Advisory Committee, Peter Cartwright is president of Cartwright Consulting Co., of Minneapolis, MN. With over 30 years in the water and wastewater treatment industry, his expertise spans membrane separation, filtration, electrodialysis, deionization and ozonation processes. Contact: 952-854-4911, [email protected] or www.cartwright-consulting.com