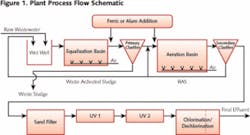
Wastewater from a U.S. research pharmaceutical facility with a large contribution from sanitary wastewater of animal and human origin was treated at a conventional activated sludge plant. The plant utilized the addition of ferric chloride to the primary clarifier for phosphorus control followed by extended aeration, sand filtration and chlorine disinfection prior to discharge to a stream (see Figure 1). The key discharge permit conditions included standard BOD5/TSS limits, ammonia and phosphorus limits of 1 mg/L each and a fecal coliform limit of 400/100ml (one sample per month).
While the plant was in consistent compliance with its discharge permit, it sought to eliminate use of chlorine for effluent disinfection. To accomplish this objective, two off-the-shelf ultraviolet (UV) disinfection units were installed, each with a nominal rated capacity satisfactory for 100% of the flow. But the UV units, even when operating in series, failed to achieve the required disinfection level (see Figure 2). Consequently, not only was the expected redundancy in UV disinfection not realized, but the plant had to continue to rely on chlorine addition for the permit compliance. The paper reports on results of the investigation into causes of the poor UV performance.
Potential interference
As indicated previously, the treatment system relied on split ferric chloride addition to both the primary clarifier and the activated sludge system for phosphorus control (see Figure 1). Due to the high influent phosphorus concentration (15-20 mg/L), a relatively high, combined dose of 60 mg/L of ferric chloride was used.
Ferric salts are known to have a negative impact on UV disinfection through a combination of several mechanisms:
• Dissolved iron molecules can adsorb UV radiation in the critical wavelength,
• Iron can be adsorbed into suspended solids and bacterial floc, where it can prevent UV light from reaching embedded target microorganisms,
• Iron salts can precipitate onto the UV system’s quartz tubes forming an adsorbing film.
Additionally, the fine suspension of hydrated ferric hydroxide and phosphate precipitate, resulting from ferric chloride application during the treatment process, may not be completely removed prior to disinfection. Such suspended solids could have a significant shielding effect, even at a relatively low nominal TSS concentration present in the filtered effluent.
Jar tests confirmed alum would be as effective in phosphorus reduction as ferric salts for this untypical wastewater, and such substitution was recommended and implemented. Still, the first two weeks of operation with alum addition didn’t bring about any visible improvement in UV effectiveness (see Figure 3). Initially, this was attributed to the fact that time, equivalent to several sludge ages, was required to “flush” the ferric chloride residue from the system. A site inspection of the treatment facility, however, revealed significant operating problems, which ultimately proved to have a significant bearing on the UV disinfection performance.
Organics removal
Prior to the site inspection, for an apparently extended period of time, the activated sludge system was operating with a very low MLSS concentration of approximately 100 mg/L. The permit limits (particularly for BOD5 and ammonia) were nevertheless met, due to the combination of several factors:
• Equalization basin was aerated and received waste sludge from the aeration basin, providing for partial biodegradation,
• Chemical addition at a high dose to the primary clarifier provided for high clarifier removal efficiency,
• Aeration basin with hydraulic retention time of three days provided subsequent biodegradation (and nitrification).
Despite an adequate effluent quality, the system was clearly not operating at optimal conditions and the activated sludge tank was reseeded. Proper sludge wasting and MLSS monitoring procedures were put into place. During the subsequent weeks the activated sludge inventory was build-up and the MLSS operating concentration was increased to the range of 3,000-4,000 mg/L.
Restoration of full biological activity resulted in a significant improvement to the UV disinfection effectiveness, as summarized on Figure 3. This Figure presents fecal coliform effluent results from the second UV lamp, together with effluent COD and UV transmittance (adsorption coefficient) data. The UV transmittance, which was initially in the 25-30% range, increased to 44-50%, which is a two-fold improvement in terms of the absorbance coefficient. This was adequate to bring the effluent from the second UV lamp into compliance. Yet this was still considerably less than the 65% level typically expected for activated sludge effluent. Basic light penetration laws demonstrate that such a difference in transmittance translates into a significant difference in available radiation intensity under process conditions. The resulting increase in power requirements would make UV less competitive than an alternative disinfection process in such applications.
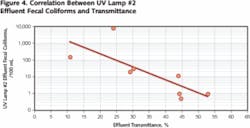
The data also documented a direct correlation between UV effectiveness (fecal coliform concentration), effluent COD concentration and UV transmittance (absorbance). Figure 4 demonstrates the expected correlation between effluent transmittance and the fecal coliform concentration.
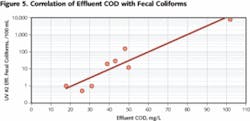
More significant is the correlation between the effluent COD and the UV disinfection performance (see Figure 5). This directly confirms that increased efficiency of biodegradation and organics removal translates into improved UV disinfection performance. It should be noted that the effluent was filtered prior to UV disinfection, thus the effluent COD is almost exclusively associated with dissolved organics.
The role of dissolved organics was further corroborated by a marked improvement of UV transmittance obtained by treatment of the effluent sample with activated carbon (see Table 1), which presumably removed the organic constituents, the primary suspects for poor UV transmittance.
The data which was collected during the evaluation provided evidence that a major impediment to UV performance is the presence of UV-adsorbing organics incompletely degraded in the activated sludge system. The chemicals used at the site, and potentially present in the wastewater, were checked against a list of chemicals (classes of chemical compounds), which are known to adsorb UV light in the critical wavelengths. This cursory investigation, though, didn’t offered a “promise” for a cost effective resolution of the disinfection problem. It was concluded that the best strategy for this relatively small facility was continuation of the practice of disinfection by chlorination.
Conclusion
The following conclusions resulted from the evaluations performed at this site:
• Optimization of the biological treatment failed to achieve typical effluent transmittance of 65%. This resulted in a significant decrease in UV disinfection efficiency,
• Poor effluent transmittance was due to UV-adsorbing recalcitrant organics,
• Investigation into the presence of the chemicals known to adsorb UV light didn’t offer a “promise” for a cost-effective resolution of the problem,
• Chlorination remained the best disinfection option for this facility.
About the Author: Jurek Patoczka is a process design specialist at Hatch Mott MacDonald, of Millburn, NJ, where he’s worked since 1989. He holds a doctorate in environmental engineering from Vanderbilt University and a master’s degree in chemical engineering from Krakow Technical University (Poland). He’s a registered professional engineer. Contact: 973-912-2541 or [email protected].