A previous issue of Water Technology outlined several important methods for improving water/steam chemistry in industrial steam generating networks to enhance equipment life and functionality.1 A partial extension of the earlier piece, this article shows how condensate and feedwater iron monitoring can alert operators and technical personnel to corrosion issues and can be used to optimize chemical treatment programs. For high-pressure steam generators that produce power and have high-purity makeup and feedwater, iron monitoring is valuable for evaluating a specific phenomenon: flow-accelerated corrosion.
Common Causes of Corrosion in Industrial Steam/Condensate Return Networks
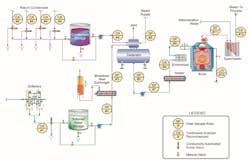
Many industrial facilities have extensive feedwater, steam, and condensate return systems whose primary material of construction is carbon steel (see Fig. 1). The focus here is on the condensate return sample points, designated as #4 in the diagram. It is common for streams from various sub-systems within a plant to be combined and then introduced to the deaerator with fresh makeup.
Given the multiple processes and products at chemical plants, refineries, petrochemical plants, and others, a variety of impurities from leaking heat exchangers or reaction vessels may be present in condensate return. Several on-line measurements are potentially suitable for monitoring condensate return, depending on the nature of the contaminants. Specific and cation conductivity (now often designated as conductivity after cation exchange [CACE]) are common, as they can provide a general indication of impurity ingress, with CACE helping account for the influence of ammonia or neutralizing amines used to adjust pH. Of course, pH itself is often a critical measurement, particularly if acidic or basic compounds might be present. At organic chemical and petrochemical plants and refineries, total organic carbon (TOC) analyses are appropriate.
Apart from these examples, certain conditions may generate significant carbon steel corrosion in condensate piping. Consider first those plants with only sodium softening for makeup water treatment, where other dissolved solids are not removed by the softener. A primary impurity is bicarbonate alkalinity (HCO3-), which, upon reaching the boiler, is in large measure converted to carbon dioxide (CO2) via the following reactions:
2HCO3- + heat → CO32- + CO2↑ + H2O
CO32- + heat → CO2↑ + OH-
The conversion of CO2 from the combined reactions may reach 90%. Carbon dioxide flashes off with the steam and, when it redissolves in the condensate, can increase condensate return acidity.
CO2 + H2O ⇔ H2CO3 ⇔ H+ + HCO3-
Although the acidity generated by this reaction has a relatively mild lower limit (minimum pH above 4), it can cause significant carbon steel corrosion in condensate return systems. The presence of dissolved oxygen (DO) can greatly magnify corrosion, generating a significant quantity of iron (mostly as iron oxide particulates) that can potentially reach part-per-million (ppm) concentrations in the condensate without effective control. Not only can this corrosion severely damage condensate piping but some corrosion products can transport to steam generators and form porous deposits on boiler tubes. The deposits may serve as sites for under-deposit corrosion that can greatly shorten boiler tube life and force units off-line. In many cases, we have found that plant personnel do not track boiler deposit buildup until failures occur, which triggers investigations. Unfortunately, by that point, the damage has already been done.
Benefits and Limits of RO Technology
Some softening makeup systems include a split-stream dealkalizer or a forced-draft decarbonator to remove most of the alkalinity, but other impurities such as silica, chlorides, and sulfate remain. Reverse osmosis (RO) technology offers a reliable option to produce makeup water with very low dissolved solids. At many power plants, RO serves as the primary demineralization step with mixed-bed ion exchange or continuous electrodeionization (CEDI) polishing as the final stage, but RO as a standalone process can easily suffice for many low-pressure boilers. The process removes the bulk (99% or greater) of all impurities, including silica, which in turn can allow for higher boiler water cycles of concentration (COC), saving costs via reduced makeup and blowdown. RO is the primary makeup treatment method used at University of Illinois at Chicago (UIC) West Co-Gen facility.
However, even with sophisticated makeup systems in place, condensate return piping corrosion can still be problematic without careful chemistry control. Iron monitoring can be a key part of any control program.
Analytical Best Practices for Condensate Iron Monitoring
A common mistake made by some plant personnel is only testing for soluble iron in condensate and feedwater streams. However, 90% or more of carbon steel corrosion products can exist as iron oxide particulates. So, dissolved iron analyses alone can grossly underestimate the amount of corrosion products. Various techniques, ranging in sophistication, are possible for total iron determinations. The least expensive are grab sample analyses. At the UIC West Co-Gen plant, personnel spot-check condensate and feedwater iron concentrations via the well-known Millipore test method, in which a known sample volume is passed through a very small-pore (0.45 micron), white filter paper, whose color is compared to standard samples after drying.
The Millipore procedure was in large measure pioneered and promoted years ago by the boiler manufacturer Babcock & Wilcox for quick calculations of feedwater iron concentrations during unit startups.2, 3 In utility boilers, virtually all of the particulates may be iron oxides, but in industrial steam generators with complex steam and condensate return networks, other impurities may exist, masking the results. So, alternative techniques may often be required.
The common colorimetric method for dissolved iron is based on the extremely sensitive ferrozine–ferrous iron complex described by Stookey.4 Ferrozine complexes with dissolved ferrous iron to form an intensely colored purple complex. The dissolved ferrous iron concentration may be determined by measuring the absorbance of this complex.
However, as stressed above, particulate iron may be important to measure. Modifications now allow for the determination of both dissolved iron and particulate iron oxides at very low concentrations utilizing a digestion-reduction-detection method.5
An advantage of this technique is the low cost of instrumentation. However, testing does require some dedicated time by personnel trained in the procedure.
Two other iron monitoring techniques that have been successfully applied, particularly in the power industry, are online particle counting and corrosion product sampling. The former can work well in high-purity waters where essentially the only particulates are iron oxides, hence the practicality for power industry applications. Corrosion product sampling (CPS) incorporates both filtration and ion exchange to capture particulate and dissolved metals, which are then analyzed to determine the metal concentrations. It is common to collect a sample for a week or so and then have the filters and ion exchange resin analyzed for results. When divided by the flow totalizer readings from the analyzer, this data provides an average concentration. Corrosion product sampling may be particularly valuable in systems that contain mixed metallurgies, most notably carbon steel and copper. Conditions that minimize iron corrosion may not be as suitable for copper. The comprehensive data provided by CPS analyzers can allow chemistry adjustments to optimize corrosion control of these two metals. A drawback is that the analyses are not performed in real time, and the data is an average over the selected period. Thus, transient events may escape detection.6
Overview of Carbon Steel Corrosion Control
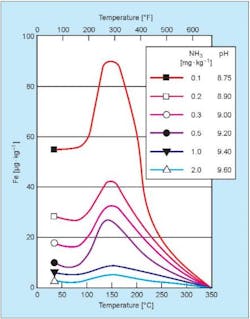
In the following section, we will briefly touch on flow-accelerated corrosion (FAC). At the time of and following the discovery of FAC in 1986, several FAC-induced piping failures at power plants have killed or injured plant personnel. While condensate/feedwater chemistry has evolved for high-pressure, high-purity-water steam generators to minimize FAC (principally by the elimination of oxygen scavengers), several other factors also influence carbon steel corrosion in general. Two of the most important are temperature and, especially, pH, as shown in Figure 2.
In accordance with pH influence, the plant staff at the UIC West Co-Gen plant diligently strive to maintain condensate and feedwater pH in a mid- to upper-9 range by neutralizing amine feed to the condensate.
And, of course, excess dissolved oxygen in condensate/feedwater networks can also cause severe corrosion, so most systems are equipped with mechanical deaerators as shown in Figure 1, which, when operating properly, should reduce the DO concentration to 7–10 ppb. A chemical reducing agent is also normally employed to further lower DO levels. For steam generators at or below a pressure of 600 psig, either uncatalyzed or catalyzed sodium sulfite (Na2SO3) is often a popular reducing agent. Use of this non-volatile oxygen scavenger adds some inorganic dissolved solids to the feedwater.
2Na2SO3 + O2 → 2Na2SO4
A common injection point is the deaerator storage tank.
Monitoring for FAC in High-Pressure Boilers
Space limitations prevent a detailed discussion of FAC in utility units, but a technical report from the Electric Power Research Institute provides full details of this phenomenon and methods of control.7 For high-pressure utility units, trace iron analysis of the condensate and feedwater may be recommended for evaluating the presence and extent of flow-accelerated corrosion.
Although FAC is a localized corrosion mechanism, the increase in iron concentration (typically at concentrations in parts-per-billion [ppb]) generated by the attack can be readily detected. Chemical treatment programs can then be adjusted to minimize FAC. A combination of a simple colorimetric total iron laboratory analysis with a sensitive laser nephelometric analyzer can provide cost effective and continuous corrosion monitoring. When properly calibrated, the nephelometric units provided by the instrument can be correlated to total iron concentration values. The iron concentration is a direct indicator of FAC.
This technique is specific to each plant and requires individual calibration at every facility.8
A question industrial plant personnel might have at this point is whether FAC can also occur in lower-pressure steam generating systems. The answer is yes, but at the lower pressures the results may not be as severe as for high-pressure units. This may be why the issue has apparently not been extensively investigated at industrial facilities yet. However, recent details from the UIC West Co-Gen plant may be of interest.9
Conclusion
The importance of iron monitoring is fortunately becoming more well known as a technique for evaluating corrosion and the efficacy of chemical treatment programs in the condensate and feedwater networks of many steam-generating system types. Methods may range from grab sample techniques to sophisticated online monitoring. Regardless, iron monitoring can be a valuable tool for establishing and maintaining proper corrosion control programs, which can, in turn, improve equipment reliability and employee safety. WT
Brad Buecker is Senior Technical Publicist with ChemTreat. He has many years of experience in or affiliated with the power industry, much of it in steam generation chemistry, water treatment, air quality control, and results engineering positions with City Water, Light & Power (Springfield, Illinois) and Kansas City Power & Light Company’s La Cygne, Kansas station. He also spent two years as acting water/wastewater supervisor at a chemical plant. Buecker has a B.S. in chemistry from Iowa State University with additional course work in fluid mechanics, energy and materials balances, and advanced inorganic chemistry. He is a member of the ACS, AIChE, AIST, ASME, NACE, the IWC Advisory Council, the Electric Utility Chemistry Workshop planning committee, and the Power-Gen planning committee.
Frank Murphy is an assistant chief engineer at UIC’s West CoGen Steam Plant. He has over 40 years of experience in steam plant operation beginning as a boiler technician in the U.S. Navy and including more than a dozen years in the power industry at Boyle Energy Services and Technology, Inc.
Ken Kuruc holds a bachelor of science degree in Chemistry from John Carroll University in Cleveland, Ohio and has been active in the Power Industry for over 26 years. In his current role, Ken provides technical support on all aspects of water quality monitoring for power, steam and industrial applications.
References
1. Buecker, B., “Protecting Water/Steam Chemistry”; Water Technology, September/October 2020.
2. Babcock & Wilcox Company, Field Services Operations, Membrane Filter Comparison Charts Procedures and Test Methods (1964).
3.Personal conversations with Frank Udo Leidich of the Advisory Council of the PPChem Journal. www.ppchem.com
4. Stookey, L., “Ferrozine-A New Spectrophotometric Reagent for Iron.” Analytical Chemistry. 1970, 42(7), pp.779-781.
5. Kuruc, K., Johnson, L. “Further Advances in Monitoring Low-Level Iron in the Steam Cycle.” PowerPlant Chemistry (now PPChem Journal) 2015, 17(2), pp. 86-89.
6. For additional information on these sampling techniques and others, see: IAPWS, Technical Guidance Document: Corrosion Product Sampling and Analysis for Fossil and Combined Cycle Plants (2014). Available from http://www.iapws.org.
7. Guidelines for Control of Flow-Accelerated Corrosion in Fossil and Combined Cycle Power Plants, EPRI Technical Report 3002011569, the Electric Power Research Institute, Palo Alto, California, 2017.
8. Kuruc, K., Johnson, L. Proc., Electric Utility Chemistry Workshop 2015, 2015 (Champaign, IL, USA). University of Illinois, Urbana-Champaign, IL, USA.
9. Buecker, B., Murphy, F.P., “Is Flow Accelerated a Concern in Co-Generation Steam Generators?”, Power Engineering, October 2020, only digital copies are available, www.power-eng.com.