Environmental Considerations in the Advancement of Cooling Treatment Technology
Cooling water systems, particularly those with cooling towers at the heart, require protection from corrosion, scaling and microbiological fouling to maximize performance. A symbolic representation of these issues and their interdependence is shown in Figure 1.
It is important to approach these mechanisms in a holistic manner, and a fourth, increasingly important, factor to consider is the environmental impact of control methods.
Environmental Concerns Around Corrosion/Scaling Treatment
Perhaps the leading environmental issue involves the often-overlapping need for improved corrosion/scaling protection with reduced phosphorus discharge in cooling tower blowdown. For four decades, the most common cooling water treatment programs for large industrial cooling tower-based systems have relied on a combination of inorganic and organic phosphate (aka, phosphonate) chemistry.
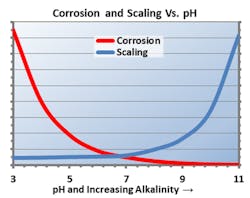
Prior to that, the most popular corrosion treatment involved feeding sulfuric acid in low dosages to remove bicarbonate alkalinity (the precursor to calcium carbonate scale), as well as using chromate for reducing corrosion potential. This treatment method generated toxic hexavalent chromium (Cr6+), and thus was phased out almost entirely in the 1980s.
The dawning realization of Cr6+ toxicity impelled a rather abrupt change to phosphate-based chemistry for both scale and corrosion prevention. These programs typically function at a mildly alkaline pH, which minimizes general corrosion. The chemistry also provides additional corrosion protection, as phosphate will react with ferrous ions (Fe2+) produced at anodic sites on carbon steel to form a reaction-limiting deposit, while Ca3(PO4)2 precipitates in the local alkaline environment at cathodic sites to inhibit electron transfer. However, even small upsets in phosphate programs can cause severe calcium phosphate fouling.
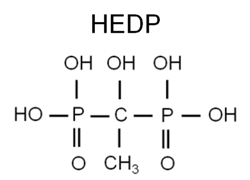
Accordingly, phosphate treatment was modified to include organic phosphates (phosphonates) with a supplemental polymer to control calcium phosphate deposition. Phosphonates attach to deposits as they form to disrupt crystal growth and lattice strength.
A common phosphate/phosphonate treatment program might include one or perhaps two of the phosphonate compounds in low mg/L dosages for primary scale control; approximately 5–15 mg/L of orthophosphate for corrosion protection; approximately 0.5–2.5 mg/L of zinc (for corrosive waters); and 5–15 mg/L of dispersant polymer for general dispersion, controlling calcium phosphate deposition, and Zn/Fe stabilization. Zinc reacts with the hydroxyl ions generated at cathodes to form a precipitate Zn(OH)2, which provides additional cathodic protection. It should be noted that zinc is also coming under tighter discharge regulations from the EPA because of its aquatic toxicity.
Phosphate/phosphonate programs are far from simple, and under- or overfeed can result in either corrosion or scale formation. Even with seemingly proper chemistry, the corrosion-inhibiting deposits are porous, and they may wash away.
Two important factors are driving an evolution away from phosphate-based chemistry. One is the increasingly problematic issue of phosphorus discharge and its effects on the generation of toxic algae blooms in receiving bodies of water. The second is substantiated results showing that alternative non-phosphate programs are more effective, both from a technical and economic standpoint, for scale prevention and corrosion protection.
Influence of Phosphate on the Natural Environment
Like nitrogen and carbon, phosphorus is a nutrient essential for all life forms. It is also often the limiting nutrient for toxic algae bloom formation, which has become increasingly common around the country.
Algae derive their carbon requirements from inorganic bicarbonate and carbonate, utilizing energy from sunlight to convert the inorganic carbon into organic carbon for cellular tissue growth. Some species of algae are also capable of “fixing” atmospheric nitrogen gas, using the nitrogenase enzyme to convert N2 into ammonia and other compounds required for the biosynthesis of nucleic acids and proteins. Common among the photosynthetic nitrogen fixing species are cyanobacteria, commonly referred to as "blue-green algae." Environmental scientists are aware of these issues, and industrial phosphorus discharge is increasingly being regulated. This factor is part of the trigger for the growing evolution in treatment chemistry to eliminate phosphorus discharge while maintaining cooling system reliability.
The Emergence of Polymer Chemistry for Scale Control
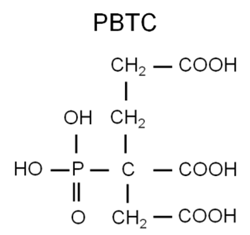
Polymer formulations containing the carboxylate group (see Figure 5) have been successfully used for decades to control calcium carbonate (CaCO3) scale in cooling water.
However, many other scaling compounds are possible, including calcium and magnesium silicates, calcium sulfate, calcium fluoride, and manganese dioxide, to name some of the most common. The need to combat these and other scale-formers has generated development of co- and terpolymers containing more than one functional group.
The polymers inhibit scale formation by two mechanisms: ion sequestration and crystal modification. Regarding the latter: even though crystals may form, the structure is very weak, and they easily wash away. A low mg/L residual is often sufficient to inhibit scale formation, but as Figure 6 suggests, the choice of polymer or polymer blend depends in large part on the cooling water chemistry. That is why comprehensive, and ideally historical, makeup water analyses are necessary, especially for new plants. Too often, project designers only receive partial water quality data, making it very difficult to select proper treatment chemistry and makeup water treatment equipment.
Corrosion Protection with Non-Phosphorus Chemistry
The chemistry outlined above has proven effective for scale inhibition, but what about corrosion control? Much progress has occurred in that area as well. One particularly effective chemical formulation is best described generically as a reactive polyhydroxy starch inhibitor (RPSI), which the author’s company developed under the trade name of FlexPro®. In this chemistry, the compound, by virtue of active sites on the molecules, attaches to the base metal and forms a protective layer. This is much more effective than reliance on the precipitation chemistry from phosphate/phosphonate programs.
Many full-scale applications of RPSI over the last decade have demonstrated the improvements. In one instance at a large industrial complex in the southeastern U.S., RPSI replaced previous polyphosphate and zinc chemistry. Carbon steel corrosion rates have been reduced from 0.2–0.25 mm/yr to 0.0025–0.0075 mm/yr. The change from phosphate/zinc to RPSI was in part influenced by severe algae formation in a clarifier and recycle pond at the plant. The removal of phosphate from the water stopped the algae formation.
Consider another example at a natural gas liquid (NGL) fractionation plant on the U.S. Gulf Coast using Wet Surface Air Coolers (WSAC®) containing galvanized cooling tubes. The open cooling water loop had been treated with a phosphate-based program, which caused considerable deposition of calcium phosphate and calcium carbonate on the hot tube surfaces and water spray nozzles. The units were first cleaned while in service by circulating a proprietary pH 6.5–7.5 cleaning agent (which included the RPSI corrosion inhibitor) for one month to avoid damaging the tubes. RPSI chemistry remained in place for subsequent operation. Figure 7 provides before and after thermal images of the tube bundles.
Heat transfer was clearly improved by this process. Similar protection is normal for other materials, including stainless steel heat exchanger tubes and equipment.
Advancements in Biofouling Control
Microbiological fouling is often the most problematic issue of all and can occur with great speed once microbes become established on cooling system surfaces. Chlorine gas was the workhorse for cooling water treatment for many years. When chlorine is added to water, the following reaction occurs:
Cl2 + H2O ⇔ HOCl + HCl
Hypochlorous acid (HOCl), is the killing agent, and it functions by penetrating cell walls and oxidizing internal cell components. The efficacy and killing power of this compound are greatly affected by pH because of the equilibrium nature of HOCl in water, as shown in Eq. 2.
HOCl ⇔ H+ + OCl-
OCl- is a much weaker biocide than HOCl, probably because the charge on the OCl- ion does not allow it to effectively penetrate cell walls. The dissociation of hypochlorous acid dramatically increases as the pH rises above 7.5, which will be shown in a later illustration. Because most cooling tower scale/corrosion treatment programs operate at an alkaline pH, chlorine chemistry may not be the best choice for some applications. Chlorine efficiency is further influenced by ammonia and organics in the water, which react irreversibly with the chemical and increase chlorine demand.
Because of safety concerns, liquid bleach (NaOCl) feed replaced gaseous chlorine at many plants, although bleach is more expensive. Bleach often contains a small amount of sodium hydroxide, so when it is injected into the cooling water stream, it raises the pH, perhaps only slightly, but if the water is alkaline to begin with, most of the reactant will exist as the OCl- ion, which can be problematic. An alternative is on-site hypochlorite generation, which has proven to be effective in several applications.
Another environmental concern with chlorine treatment is the potential formation of halogenated organics. Some of these compounds are suspected carcinogens, and guidelines have been formulated that restrict the concentration of these substances. This issue has only grown in importance, given the diminishing availability of fresh water supplies for new industrial plants, including those for power production. In some areas of the country (California is a notable example), local regulators are mandating the use of treated municipal wastewater plant effluent as industrial facility makeup. These supplies can introduce a variety of impurities to the cooling water, including organics that can react with chlorine.
Chlorine Alternatives
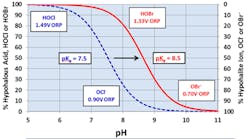
As has been noted, the killing power of chlorine falls off with a rise in pH, which is problematic given that most scale/corrosion inhibitor programs operate in a mildly basic pH range. A popular solution to this difficulty is bromine chemistry, where a chlorine oxidizer (bleach is the common choice) and sodium bromide (NaBr) are blended in a makeup water stream and injected into the cooling water. This chemistry produces hypobromous acid (HOBr), which has similar killing powers to HOCl but functions more effectively at an alkaline pH. Figure 8 compares the dissociation of HOCl and HOBr as a function of pH.
Note that at a pH of 8.0, 80% of the HOBr remains undissociated, while only approximately 25% of the HOCl is still intact.
Beyond this fundamental oxidizing chemistry, numerous facilities such as refineries, chemical plants, steel and paper mills, food and beverage plants, etc., often have cooling systems with waters containing elevated organics, nitrogen species, or other impurities that severely inhibit the performance of conventional oxidizers. As previously noted, most cooling tower systems operate at a pH above 8.0, which significantly reduces the effectiveness of chlorine.
ChemTreat researchers have developed, or improved upon, alternative oxidizing biocides that may perform much more effectively in difficult cooling waters. One such alternative is based on monochloramine (NH2Cl) chemistry. Monochloramine is a weaker oxidizer than chlorine, bromine, or chlorine dioxide, but research and operating experience show the compound is more effective at penetrating the protective slime layer of bacterial colonies. Strong oxidizers are typically consumed by this slime, but the monochloramine moves through the layer to attack the microorganisms underneath.
Another option is a specialty solution of chlorine dioxide (ClO2). This compound is a selective oxidizer, but even though it is chlorine-based, it does not react with ammonia and reacts less vigorously with some organics than chlorine. Furthermore, the compound is not influenced by pH, unlike chlorine. On-site chlorine dioxide generation is required, as large quantities of chlorine dioxide cannot be safely stored in containers or tanks. However, modern production methods include more safeguards and safety checks than past technologies.
For those plant personnel who still wish to use bleach (sodium hypochlorite), but whose cooling systems face at least some of the challenges mentioned above, SurfClean™ technology may be of significant benefit. These products contain a patent-pending combination of halogen stabilizer and bio-penetrant. The former, as its name implies, stabilizes the chlorine in solution and provides a controlled release to more effectively attack microbes. The bio-penetrant greatly aids this process by destabilizing protective slime layers to allow the oxidizer greater access to the underlying organisms. These products do not carry microbial control claims and thus are not subject to the tight restrictions of other compounds.
Conclusion
This article provided an overview of several of the most important factors influencing modern cooling water chemical treatment. While environmental issues are certainly drivers for this evolution, advancements in technology to provide better protection and reliability of cooling systems have also played a key role.
As you know, all systems are different. Like all other technologies, due diligence is necessary to determine the feasibility for utilizing methods. Always consult your equipment manuals and guides. WT
About the Author: Brad Buecker is senior technical publicist with ChemTreat. He has many years of experience in or affiliated with the power industry, much of it in steam generation chemistry, water treatment, air quality control, and results engineering positions with City Water, Light & Power (Springfield, Illinois) and Kansas City Power & Light Company’s La Cygne, Kansas station. He also spent two years as acting water/wastewater supervisor at a chemical plant. Buecker has a B.S. in chemistry from Iowa State University with additional course work in fluid mechanics, energy and materials balances, and advanced inorganic chemistry. He is a member of the ACS, AIChE, AIST, ASME, NACE, the IWC Advisory Council, the Electric Utility Chemistry Workshop planning committee, and the Power-Gen planning committee.