Brine mitigation for the mining and food and beverage industries
There are a few common facts regarding water for industries like mining, food and beverage, textile and pulp and paper, including:
- Water is essential during virtually every stage of production in numerous industrial processes.
- Water scarcity and stringent environmental regulations push the industry to seek more economical water recycling solutions that enable maximum water reuse and minimal effluent discharge.
- Brine minimization and mitigation measures — and more specifically commercially viable and sustainable solutions — are therefore gaining more momentum than ever before.
Industries like mining, textile, pulp and paper, and food and beverage are particularly hard hit by these challenges, as water is a critical component in almost every aspect of production. According to Reuters, the world’s top mining companies have recently been warned that assets will be stranded and investors will walk away unless they address water scarcity in key mining regions such as Africa, Australia and Latin America. It has become critical for mining companies to properly treat mining effluent, as well as maximize in-house water recycling. These challenges are not restricted to just mining companies — many other industries are in search of efficient and cost-effective ways to treat effluents as well.
The pressure to minimize brine is on
Brine is a byproduct of many industrial processes such as reverse osmosis (RO) desalination, and it can be found in a wide variety of applications, from generating high-purity water for industrial use to treating various industrial effluents. The latter can include blowdown water of power plant cooling towers, produced water from oil and gas operations, acid mine drainage (AMD), pulp and paper mill effluent and waste streams from food and beverage processing. The challenge is that along with diluted salts, these types of effluents can include organic contaminants, industrial reaction byproducts and heavy metals due to corrosion.
In mining, for example, where effluent-related issues seem to have the most considerable impact, several potential sources of water pollution exist, including: process wastewater, seepage from tailing facilities, waste dumps, metal-concentrate processing facilities, ponds and other mine facilities. Consequently, one of the major environmental hazards recognized in the mining industry is AMD, characterized by neutral to low pH and heavy metal content.
These environmental issues, which all stem from brine disposal, are not only challenging to treat, but are also cost-intensive tasks faced by industries and municipalities around the world.
Costly endeavors
Brine treatment may involve the removal of suspended solids by varying means of filtration (media or membrane-based), biological treatment for the removal of organic contaminants and dissolved salt removal from the waste stream by using ion exchangers, or more commonly, RO. In cases where zero liquid discharge (ZLD) or minimum liquid discharge (MLD) solutions are required, the treatment process will most likely include additional evaporation processes such as mechanical vapor recompression (MVC) and thermal crystallizers.
Thermal evaporation processes are the most widespread for brine treatment because they enable the highest degree of concentration, as high as solid salt. They also produce the highest purity effluent that can reach distillate quality. Energy consumption of thermal systems is excessively high, which makes thermal solutions expensive and sometimes financially unattainable.
When it comes to the removal of dissolved salts, although similarities to seawater or brackish water desalination exist, industrial brine treatment usually contains unique combinations of dissolved ions, necessitating specific processes and equipment.
Existing solutions
Meeting the challenges above with the implementation of a conventional RO system is not easy due to potential fouling by organic contaminants, but a major attribute is the damage to the RO membranes by scaling for sparingly soluble salts.
Existing solutions designed to address brine minimization also pose several drawbacks, which limit their use in industrial and mining water treatment applications and may even prevent some from being economically feasible. For example, conventional systems might be limited in cases where feedwater varies in quality and variable recovery rates are required, or in cases of supersaturation of sparingly soluble salts.
As a result, significant efforts are being made by the global water industry to come up with a solution that will combine the cost-effectiveness of RO with a technology that supports maximum recovery compared with currently available solutions.
Water chemistry
Sulfate, typically the predominant ion in mine water, is formed by the oxidation of sulfide-bearing ores (often pyrite), and is primarily controlled by a combination of technologies: membrane separation to remove salts from the water and salt precipitation to remove sulfate. However, it has become difficult to address the recent demand for low sulfate concentration using existing technologies while keeping costs low.
Putting the factor of cost aside, the main challenge with minimizing quantities of mining wastewater, such as AMD, is the chemistry of wastewater. As the concentration of salts in wastewater rises during a treatment process, there is an increased risk of precipitation of salts on all available surfaces such as membranes and pipes. Therefore, new and cost-effective technologies are required to address environmental and regulatory challenges while mitigating the risk of increased precipitation.
System solution
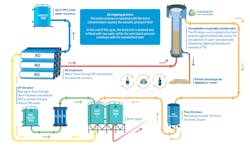
A recently developed technology, called MaxH2O DESALTER, is an RO system with an integrated salt precipitation unit. The system overcomes the limitation of wastewater chemistry and provides a unique solution for difficult industrial wastewater and brackish water, which require treatment to provide high recovery.
This system eliminates various challenges such as scaling by sparingly soluble salts, as well as organic fouling and biofouling. It has been successfully tested for minimizing the volume of brackish water brine and industrial wastewater concentrates, as well as for treating water with a high sulfate content to meet strict discharge regulations. This technology overcomes the historically inherent limitation of the recovery process when treating these concentrates (the risk of calcium sulfate, calcium carbonate and silica scaling).
With MaxH2O DESALTER, brine is circulated in a high velocity through the RO system in a semibatch process. In each cycle, brine concentration is gradually increased alongside the saturation level of the sparingly soluble salts. As brine exits the RO membrane, it flows through a pellet reactor. This stage enables precipitation of some of the supersaturated salts to a level that allows brine to go through an additional RO cycle without the risk of scaling. This semibatch process continues until brine concentration reaches the osmotic pressure limit and is then ejected out of the system.
By using a semibatch process, the flow through the membranes increases, resulting in high cross-flow velocities inside the feed-brine spacer. The result reduces the effect of concentration polarization and the risk of scale formation.
This development also addresses other challenges such as biofouling due to the changes in the brine osmotic pressure throughout the process without the use of additional chemicals.
Producing pellets of more than 90% dry solids content means the system eliminates the need in further sludge dewatering. Depending on the effluent requirements, the obtained RO brine stream can be partially or completely blended with an RO product stream, pushing the total recovery rate of the system even further.
Conclusion
This solution benefits industrial brackish water reverse osmosis (BWRO) applications such as cooling towers blowdown treatment, diverse mining applications, petrochemical and power plant effluent. So far, it has been deployed successfully in several locations around the world, treating impacted wastewater with high levels of metals and sulfate, and demonstrating a recovery rate of up to 98%.
Implementation of this technology reduces operational costs by decreasing chemical consumption and diminishing the amount of sludge for discharge. Investment costs are cut due to the high recovery achieved. MaxH2O DESALTER’s ability to practically eliminate the recovery limitation of water chemistry while operating RO at high velocity with inherently high shear forces allows the RO unit to meet its maximum production and recovery potential, overcoming chronic scaling issues and delivering operational efficiency.