The Scoop on Selenium: Exploring Sources, Fate and Transport of Se in Oil Refining
The chemistry of Se reactions can require complex treatment considerations due to the speciation of Se into numerous compounds as it travels through various refinery manufacturing processes. With NPDES permit levels in the low part per billion (ppb) range, treatment for Se reduction can become even more complex.
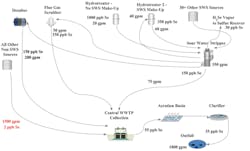
A common configuration of a medium-sized oil refinery water system as it pertains to Se fate and transport can be seen in Figure 1.
The following points summarize important considerations in remediating Se:
- Almost all of the incoming Se to an oil refinery enters with the crude oil. The contribution from refinery supply water is negligible.
- The Se in the crude is in the form of various organoselenides. Its chemistry is similar to sulfur, and in general, Se will follow the pathways of sulfur. Likewise, the amount of Se in crude is usually proportional to sulfur, at an estimated ratio of 1:1000.
- As Se travels through various refining processes, it accumulates in the sour water system.
- The bulk of organoselenides in the crude will travel with the oil phase upon passing through the desalter. The crude contribution to the desalter Se effluent water will be negligible. It is the desalter wash water originating from the sour water stripper (SWS) that is responsible for the Se contribution to the desalter effluent.
- Most of the organoselenides convert to hydrogen selenide, particularly in the hydrotreaters.
- The FCC Unit contributes significant thiocyanates to the sour water system. In the SWS, thiocyanates react with hydrogen selenide to create selenocyanates, which are the predominant species remaining in the water in the SWS bottoms.
- Most of any unreacted hydrogen selenide in the SWS goes overhead as a vapor to the sulfur recovery unit.
- Some elemental Se formed by high temperature breakdown of other species that stay with the heavier oil fractions upstream, particularly in the vacuum unit, is removed from the refinery via asphalt manufacturing.
- In evaluating additional treatment alternatives, it is apparent that wastewater treatment plant (WWTP) end-of-pipe options could be cost-prohibitive due to low concentration versus high flow rate. Instead, treatment for low-flow high-concentration isolated streams is a more cost-effective approach, both for capital investment and for operations and maintenance (O&M).
- There are significant correlations between changes in the levels of Se in refinery wastewater streams versus the type of crude being run at that time. This crude slate design can affect Se source control.
- Certain designs of wastewater reuse systems can inherently solve Se removal problems. For example, by treating for the reuse of SWS bottoms as boiler feedwater, the remediation of Se is inherent within that process (see "Sweet Plan for Sour Water, IWW, Nov. 2013").
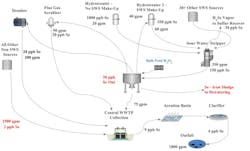
The iron selenite design (see Fig. 2) is well established but is known for very high OPEX due to the high usage of hydrogen peroxide and the sludge disposal costs.
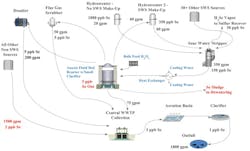
The anoxic reduction design (see Fig. 3) has shown a marked improvement over the co- precipitation process by increasing Se removal rates and by reducing OPEX via the elimination of iron addition, which is a major contributing factor to the Se sludge volume and a hazardous waste designation.
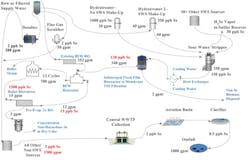
Independent of Se removal, wastewater reuse has become more widespread as advancements in technology have developed in recent years. In the case of a well-controlled SWS process in refining, the resulting bottoms are actually too valuable to be used for such purposes as desalter wash. In these cases, once residual NH3, H2S and very short chain hydrocarbons are removed, the equivalent of steam condensate quality supply water within the context of use as boiler feedwater is a result. All of these contaminants are very biodegradable, and with the advent of advances in biological wastewater process control, such a treatment system can provide reliable high-quality boiler feedwater without needing further pretreatment (see Fig. 4).
An efficient SWS will not contain any significant levels of cations with scaling potential. The relatively low level of anions present in this stream poses no threat for boiler scaling, and in fact, some resulting species, such as NO3, will actually help protect the boiler from corrosion mechanisms such as caustic embrittlement. The treatment cost comparison using this approach would be less than one-fifth the cost of reverse osmosis (RO) or demineralization pretreatment, which this produced stream would bypass on its way directly to the deaerator. Further, significant energy savings can be captured via the heat content of this SWS water, thus reducing the energy needed to heat the water on the way to the deaerator.
Therefore, if a capital investment is made at the SWS for the purpose of Se removal and permit compliance, for a little extra cost two goals could be accomplished simultaneously:
- Cutting the cost of a selenium removal process to a very small fraction of what it would be as a standalone treatment system.
- Recouping the investment quickly by offsetting the cost of RO supply water pretreatment with the new boiler feedwater supply produced from the SWS bottoms. This savings will approach 80 percent in pretreatment costs when adding in the fuel savings by utilizing the recovered heat at the deaerator. Of course, this design also removes close to 20 percent of wastewater flow to the central treatment plant and over 50 percent of the ammonia load. Further, there are usually numerous steam condensate streams that could be rerouted from the central WWTP to this same SWS bottoms treatment system, which would dramatically increase the return-on-investment.
With regard to sludge disposal, selenium is one of eight metals listed and regulated by the Resource Conservation and Recovery Act (RCRA). The obvious question is whether this sludge will be listed as hazardous. This can only be determined by the performance of a site- and process-specific Toxicity Characteristic Leaching Procedure (TCLP) test. This test must be conducted regularly on an operating process for ongoing determinations. The TCLP lower limit on Se for a hazardous listing is 1,000 ppb.
This test must be run each time a process changes internally or transfers to another process. Usually it is run on centrifuged solids from processes where the concentration of solids is high, for example in sludge dewatering operations. In sludge generation systems where centrifuged solids would be relatively low, such as in clarifiers and in sedimentation, this test is usually conducted on a non-filtered sample. In all cases, various stabilizing chemicals can be added to a process in order to minimize the amount of hazardous contaminant that would leach out of the solids into an aqueous phase during the TCLP test.
About the Author: David Kujawski is vice president of Refinery Water Engineering and Associates in Nederland, Texas. He has 32 years of experience in water and waste treatment in over 200 industrial plants and 46 oil refineries. Kujawski holds degrees in environmental engineering, chemistry and marketing and over his career has held positions at Nalco, Betz-Dearborn, Baker-Petrolite, US Filter-Siemens, Chevron El Segundo Refinery, Sybron Biochemical, and Ashland Oil.