The importance of industrial water and steam treatment, Part 7
The previous installments in this series outlined important fundamentals of steam generation makeup treatment and process chemistry control. Maintaining proper chemistry is difficult to impossible without accurate, analytical monitoring of the entire steam-generating network. In recent decades, on-line monitoring has become quite reliable for chemistry tracking, with the primary benefit being that on-line instrumentation provides near instantaneous data upon which plant operators and technical personnel can act to protect boiler systems. Bench-top analyses are important as backup to on-line monitors, but we will mostly focus on continuous analyses in this installment.
Sampling points and monitoring parameters
Keeping close track of conditions in high-pressure steam generators is critical, as even seemingly minor upsets or off-spec chemistry control can be greatly magnified in the high-temperature, high-pressure environment. However, the monitoring of low-pressure industrial systems is also important.
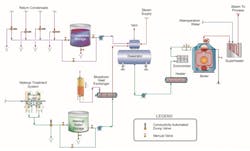
Utility steam generator design has evolved significantly from the large coal-fired plants that dominated power generation in the last century. Apart from renewable sources and simple-cycle combustion turbines, the primary replacement for the many retired coal units has been combined cycle power. Accordingly, the following sections will mostly focus on chemistry monitoring for combined cycle heat recovery steam generators (HRSGs). Space limitations prevent a detailed discussion of the nuances in these systems, but details may readily be found in References 1, 2 and 3.
The samples of primary importance for utility steam generators are:
- Makeup treatment
- Condensate pump discharge
- Feedwater/economizer inlet
- Boiler water
- Saturated steam
- Main and reheat steam
For co-generation and industrial units, condensate return typically has some impact on chemistry. Several monitoring issues in this regard are addressed later in this article.
Makeup treatment system
In the absence of major leaks, the water/steam network in a utility unit is a nearly closed system with just small losses. Even so, some makeup is always required. For modern combined cycle units, the most common core makeup treatment process is reverse osmosis (RO) followed by either mixed-bed ion exchange (MBIX) or electrodeionization (EDI) to “polish” the RO effluent. RO units typically include numerous instruments to monitor system performance, including pressure, temperature, flow and specific conductivity. These measurements and others allow operators to track RO performance, identify upsets, and schedule membrane cleanings. Additional details are available in Reference 4.
Common guidelines for the final effluent quality from a high-purity makeup system are:
- Specific conductivity: ≤0.1 µS/cm
- Silica: ≤10 µg/L
- Sodium: ≤2 µg/L
These measurements ensure that high-purity water is constantly being distributed to the steam generators. Excluding an instrument malfunction, an increase in any value indicates that either the MBIX resin has reached exhaustion or that a problem has occurred in the EDI unit. Prompt corrective action is necessary.
Case history: At a power plant where RO had been installed ahead of an existing ion exchange (IX) unit, the IX unit remained in place to polish the RO effluent. On one occasion, a malfunction of the anion resin regeneration process put caustic (NaOH) regenerant into the makeup water. The excess caustic raised the boiler water pH above 11. Some caustic quite possibly also found its way directly to the main steam via superheat/reheat attemperation sprays. Caustic can be extremely corrosive at the high temperatures and stress loads in superheaters and turbines.
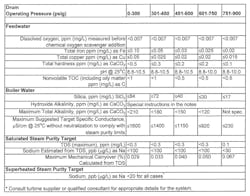
Makeup guidelines are considerably less stringent for low-pressure boilers, and Figure 1 is an extract taken from the recent revision of the American Society of Mechanical Engineers (ASME) industrial boiler water guidelines. This extract provides insight on impurity limits for low- to medium-pressure water tube industrial steam generators.
Note the low feedwater hardness levels for all cases. Too often at industrial plants, the focus is so intense on process chemistry and engineering that makeup water treatment is neglected. Many cases are known where softening unit malfunction has allowed frequent or excessive hardness leakage into the makeup. The result is boiler tube deposits that can cause under-deposit corrosion and overheating failures, along with loss of efficiency.
Bench-top analytical techniques for hardness monitoring have been around for ages, but on-line instrumentation has emerged for continuous measurements.
Case history: The author was once employed at a chemical plant where sodium softening was required treatment for the makeup feed to the primary chemical process. (At that time, the plant imported steam from an adjacent facility and had no on-site boilers.) To minimize chemistry upsets and quality control, the plant’s water/wastewater treatment group upgraded hardness monitoring capabilities from grab-sample, bench-top technology to an on-line analyzer.
Condensate pump discharge (CPD)
For utility steam generators, the primary location for potential contaminant ingress is the condenser, unless the unit is equipped with an air-cooled condenser. Leaks in water-cooled condensers allow cooling water to infiltrate the high-purity condensate and introduce many impurities, including hardness, chloride, sulfate and silica. These and other contaminants, when subjected to the harsh environment in the steam generator, may cause severe deposition and corrosion. A condensate polisher will provide a buffer against contaminant ingress, but polishers are often not considered for drum units, in large measure to reduce project capital cost, but also because operation and maintenance require additional staff. Accordingly, on-line instrumentation is critical for quick detection and corrective action of condenser tube leaks.
Recommended core CPD analyses for power units are (1, 2):
Sodium monitoring is quite effective for detecting condenser tube leaks. With a tight condenser, sodium levels in the condensate are normally very low (< 3 µg/L), and in many cases less than 1 µg/L, so even a small condenser tube leak is noticeable. Continuous monitoring allows prompt reaction to impurity ingress.
Cation conductivity is often now referred to as “conductivity after cation exchange (CACE)” to establish that the sample is routed through a cation exchange column to replace all cations, i.e., ammonium, sodium, calcium, etc., with hydrogen ions. This creates a dilute acid solution of primarily trace amounts of chloride and sulfate ions, whose conductivity is then measured. As with sodium, a rise in CACE indicates impurity in-leakage, although this measurement is influenced by carbon dioxide ingress, say from increased air in-leakage at the condenser. Thus, becoming increasingly popular is degasified CACE, which utilizes either a re-boiler or nitrogen sparging compartment to remove CO2.
CACE can also be greatly influenced if the feedwater pH-conditioning program employs an alkalizing amine, e.g., ethanolamine, cyclohexylamine, morpholine, etc., as a blend with ammonia or as a standalone treatment. The portion of alkalizing amine that carries over into superheaters and reheaters decomposes at the high superheat temperatures into small chain organic acids and CO2.6 The acids pass through cation exchange columns and artificially increase CACE. They can also lower the pH of the condensate.
Case history: The author consulted for a time at a coal-fired power plant, in which a blend of cyclohexylamine and morpholine served as the feedwater pH-conditioning agents. Typical CPD CACE values ranged from 1 to 4 µS/cm, which were much higher than the 0.3 µS/cm target value above. CACE was useless for detecting condenser tube leaks. Also, the inorganic acids that carried over into the condenser consistently kept the condensate pH slightly below 9; a key issue regarding the potential for carbon steel flow-accelerated corrosion (FAC). After plant personnel installed a corrosion product sampler at our request, the iron data clearly suggested FAC in the condensate system ahead of the deaerator.
Dissolved oxygen (D.O.) analyses are important for monitoring condenser air in-leakage. A sudden increase in dissolved oxygen may indicate a mechanical failure at or near the condenser, which allows excess air to enter the system.
A parameter not typically monitored on-line in power units, but which can be important is total organic carbon (TOC). For utility steam generators, the normal TOC limit in the CPD sample is 100 ppb.
Feedwater/economizer inlet
Unlike the conventional coal-fired units of the past, heat recovery steam generators (HRSGs) do not have the string of feedwater heaters (often tubed with copper alloys) from the CPD to the economizer. Thus, the piping from the condenser to the economizer, and the economizer(s) are all carbon steel. Accordingly, the recommended feedwater conditioning program is all-volatile treatment oxidizing (AVT(O)), in which ammonia is the pH-conditioning agent and where the dissolved oxygen introduced at the condenser is allowed to remain, with no oxygen scavenger/reducing agent feed. (The author discussed AVT(O) chemistry, which is designed to control FAC, in greater depth earlier in this series.7)
Recommended core FW/EI analyses for power units* are:
Maintaining pH within the range shown helps to minimize both general corrosion and FAC. However, direct pH measurement of high-purity water can be tricky. Algorithms have been developed that calculate pH from conductivity measurements to provide more accurate results, provided that the pH is reasonably close to the recommended range. In addition, specific conductivity (S.C.) in high-purity water directly correlates with the ammonia concentration, and thus S.C. measurements offer better control of ammonia feed than pH.
Note also the recommended D.O. concentration of 5-10 µg/L, which some industry experts suggest should be higher (perhaps 20-30 µg/L) to provide the necessary carbon steel passivation from an AVT(O) program. The extensive design of multi-pass HRSGs can complicate this issue.
Not shown on the core list, but which is a key measurement to determine the effectiveness of any condensate/feedwater treatment program, is iron.8 To briefly review, a properly implemented feedwater treatment program should keep iron concentrations below ≤2 µg/L. Usually, however, 90 percent or greater of iron corrosion products are particulate in nature, which must be accounted for by the analytical procedure. Several methods exist to evaluate total iron concentrations, including:
- Continuous particulate monitoring
- Corrosion product sampling
- Grab sample analysis
- On-line nephelometry
For plant personnel interested in an inexpensive approach, improved bench-top methods are available, in which, with proper sample handling and treatment, iron measurements down to 1 ppb are possible. This method can provide near real-time data of carbon steel corrosion severity, although on a snapshot basis.
Condensate return
Steam recovered as condensate from process heat exchangers may contain substantial impurities, ranging from mineral salts to particulates to organic compounds. Any of these materials can potentially be quite problematic.
Case history: The author once visited an organic chemicals plant where condensate return represented 85% of the boiler makeup. Substantial organic contamination of the return condensate caused drum foaming, impurity carryover to steam and subsequent reoccurring failures of the superheaters in four, 550 psig steam boilers. Tests by the company’s water treatment vendor indicated periodic TOC excursions above 200 mg/L. Compare that value to the recommended limit of 0.5 mg/L shown in Figure 1. It was no surprise that foam formation was a serious issue in the boiler drums.
In cases like this, it is important to identify potential impurities during plant design and incorporate a method or methods to control impurity transport to the steam generators. Corrective measures may include condensate polishing or condensate dumping in the event of an upset. On-line instrumentation at strategic locations can provide valuable data on chemistry upsets and performance of polishers, if they have been included in the design.
Boiler/HRSG evaporator water
Boiler water sampling is critical for two primary reasons. First, poor chemistry control and/or poor monitoring can allow unacceptable carryover of excess impurities to the steam. Secondly, the effects of impurity ingress or poor chemistry are magnified by the high temperatures and pressures in these circuits.
Case history: An 80 MW power generating unit supplied by a 1,250 psig coal-fired, cyclone boiler had just been returned to service from a scheduled autumn outage. Laboratory personnel discovered that a condenser leak was allowing contaminants to enter the system, such that condensate total-dissolved-solids (TDS) concentrations at times reached 0.75 ppm. Although the lab staff requested that the boiler be taken off-line immediately, the operations managers refused due to load demand issues. The boiler was on congruent phosphate2 control at that time, so the lab staff increased monitoring frequency and attempted to maintain phosphate and pH levels within recommended guidelines. After approximately three weeks, operators discovered the source of the leak and corrected the problem. Two months later, boiler waterwall tubes began to fail with alarming frequency. The unit came off-line numerous times for tube repairs, and in at least one instance had only been back on-line for a few hours when another tube failed. The failures happened so regularly that plant management scheduled an emergency tube replacement during the upcoming spring outage. The repairs cost over $2,000,000. The mechanism attributed to these failures was under-deposit corrosion/hydrogen damage caused by excessive sludge and scale formation.
For most conventional drum units and for the intermediate- and high-pressure evaporators of an HRSG, a solid alkali treatment (tri-sodium phosphate or perhaps caustic) is utilized to maintain pH somewhere within a 9 to 10 range and to mitigate the effects of chloride and sulfate that could potentially enter from a condenser tube leak.
Recommended core analyses for boilers on phosphate treatment include:
Comment is necessary regarding phosphate. Apart from pH control, solid alkalis counterbalance the potential under-deposit corrosive effects of chloride and sulfate. Tri-sodium phosphate (Na3PO4) is the core boiler water treatment chemical for many drum units. However, phosphate dosing control can be difficult due to the compound’s reverse solubility above 300o F. Phosphate drops out of solution (hides out) as boiler load increases, and then returns to solution as load decreases. Given that many combined cycle and conventional units now operate in cycling mode to follow load from renewables, hideout can present significant challenges. Some plant personnel, especially in the power industry, have switched to caustic (NaOH) feed to eliminate phosphate hideout, but great care is required with these programs to prevent caustic gouging of waterwall tubes.
Increasingly recognized is the importance of boiler water iron monitoring; HRSG target value <5 µg/L. The LP circuit and some IP locations in a multi-pressure HRSG can suffer from the phenomenon known as two-phase FAC. Additional details are available in the references, but iron monitoring is important to ensure that proper chemistry is being maintained in these circuits.
Steam
Steam purity measurements are very important, particularly if the steam drives a turbine. Contaminant deposition on turbine blades can lead to corrosion and eventually possible blade failures, which represent a potentially catastrophic situation with the turbine spinning at 3,600 revolutions-per-minute (rpm). The following table lists the saturated, main, and reheat steam target values that apply to all high-pressure units.1,2
Sodium provides a direct indication of salt or sodium hydroxide carryover with the steam. Salts, and particularly chloride salts, will settle in the last rows of the low-pressure turbine, where they can cause pitting and subsequent stress corrosion cracking (SCC) and corrosion fatigue (CF) of turbine blades and rotors. Sodium hydroxide carryover is a very serious issue, as caustic can quickly induce SCC of turbine blades.
CACE provides a surrogate measurement of chloride and sulfate carryover, and 0.2 µS/cm has been a long-time guideline for turbine manufacturers. However, this CACE does not match the recommended target value for both impurities of 2 µg/L. A relatively new instrument has emerged on the market that allows analyses of chloride and sulfate down to a 0.1 ppb level.
It has long been known that steam-borne silica will precipitate on turbine blades. While the compound is not corrosive, it can influence turbine aerodynamics and reduce efficiency. Many guidelines recommend a 10 or 20 µg/L target value. Because silica can carryover vaporously in steam, boiler water silica monitoring is also common to keep track of concentrations and proactively inhibit carryover.
Recommended are several steam sampling points in power generating units. These are saturated and main/reheat steam. Main and reheat steam are the most important, as they provide data on impurities directly on the path to the turbine. Main and reheat sections are also the entrance point for attemperator sprays. Measurement of saturated steam is important on a periodic basis to check for mechanical carryover from steam drums. Damaged or failed steam separators are a common cause of mechanical carryover.
Sample extraction and conditioning
Proper design and installation of sample taps, sample lines and sample conditioning equipment are critical for accuracy. Reference 9 offers valuable information on this topic.
Conclusion
Without proper and comprehensive sampling, trying to correctly operate any steam-generating unit from a chemistry perspective is like flying blind. This installment provided an overview of important issues, with many additional details provided in the references.
References
1. International Association for the Properties of Water and Steam, Technical Guidance Document: Volatile treatments for the steam-water circuits of fossil and combined cycle/HRSG power plants (2015). Available from http://www.iapws.org.
2. International Association for the Properties of Water and Steam, Technical Guidance Document: Phosphate and NaOH treatments for the steam-water circuits of fossil and combined cycle/HRSG power plants (2015). Available from http://www.iapws.org.
3. International Association for the Properties of Water and Steam, Technical Guidance Document: Instrumentation for monitoring and control of cycle chemistry for the steam-water circuits of fossil and combined cycle/HRSG power plants (2015). Available from http://www.iapws.org.
4. Water Essentials Handbook (Tech. Ed.: B. Buecker). ChemTreat, Inc., Glen Allen, VA, 2023. Currently being released in digital format at https://www.chemtreat.com/.
5. Consensus on Operating Practices for the Control of Feedwater and Boiler Water Chemistry in Modern Industrial Boilers, The American Society of Mechanical Engineers, New York, NY, 2021.
6. Shulder, S. and Buecker, B., “Remember the 3Ds of Alkalizing Amines: Dissociation, Distribution, and Decomposition”; PPCHEM Journal, 25/2023 – No. 1.
7. Buecker, B., “The importance of industrial water and steam treatment, Part 4”, Water Technology, December 2023.
8. Buecker, B., “The importance of industrial water and steam treatment, part 4.5”; Water Technology, January 2024.
9. Sentry Equipment Corporation, Steam Sampling 101: Basics and What Really Matters. Available at www.sentry-equip.com.
About the Author

Brad Buecker
Brad Buecker currently serves as Senior Technical Consultant with SAMCO Technologies. He is also the owner of Buecker & Associates, LLC, which provides independent technical writing/marketing services. Buecker has many years of experience in or supporting the power industry, much of it in steam generation chemistry, water treatment, air quality control and results engineering positions with City Water, Light & Power (Springfield, Illinois) and Kansas City Power & Light Company's (now Evergy) La Cygne, Kansas, station. Additionally, his background includes 11 years with two engineering firms, Burns & McDonnell and Kiewit, and he spent two years as acting water/wastewater supervisor at a chemical plant. Buecker has a B.S. in chemistry from Iowa State University with additional course work in fluid mechanics, energy and materials balances, and advanced inorganic chemistry. He has authored or co-authored over 300 articles for various technical trade magazines, and he has written three books on power plant chemistry and air pollution control. He is a member of the ACS, AIChE, AIST, ASME, AWT, CTI, and he is active with Power-Gen International, the Electric Utility & Cogeneration Chemistry Workshop, and the International Water Conference. He can be reached at [email protected] and [email protected].