A critical issue at coal-fired power plants — those remaining and many of those that have shut down — is how to remove selenium from wet scrubber effluent and ash pond wastewater. However, industries such as mining, oil refining, and others face similar difficulties where wastewaters from material extraction or process treatments contain selenium. Heretofore, selenium removal has been capital intensive, as the standard removal methods involved microbiological treatment with large and complex systems. This article outlines fundamental concepts of a new process that can remove selenium via efficient physical-chemical techniques.
The Difficulties with Selenium
An examination of the periodic table reveals that selenium lies immediately below sulfur in the chalcogens group of the table. Selenium is a non-metallic element that can bioaccumulate at concentrations that may cause toxicity in humans, fish, and wildlife. It is a trace element that has biochemical properties similar to those of sulfur and is widely distributed in rocks, soils, and living organisms.1
Selenium can occur in four different oxidation states: selenide (2-), elemental selenium (0), selenite (4+), and selenate (6+). In general, selenate (Se6+) has a high solubility and is the most mobile in water. Selenite (Se4+) is soluble in water, but its strong adsorption affinity to soil particles greatly reduces its mobility. Elemental selenium (Se0) exists in a crystalline form and is usually incorporated in soil particles. Selenide can occur as metal selenides (similar to metal sulfides), which tend to be deposited in bottom sediments, or as organic selenides (primarily as dimethylselenide) through methylation and volatilization.
Selenium is often an analog to sulfur in many biochemical reactions and can cause problems in animals if it replaces sulfur in some metabolic pathways. Like sulfur, it readily bonds with oxygen, with the common anions being SeO32- (selenite) and SeO42- (selenate). While trace amounts of selenium are an essential human nutrient, in the concentrations that often exist in mine wastewaters, refinery desalter effluent, and coal-fired power and industrial plant ash ponds, the selenium can be quite toxic.
Selenium (Se) readily bioaccumulates through the food chain at levels that can cause adverse effects on higher-level aquatic life and wildlife, including fish and birds that prey on fish and invertebrates. Toxicological effects of selenium on wildlife include lowered reproduction rates (e.g., impaired hatching), shortened life spans, stunted growth, and impaired immune response.2
Thus, the United States Environmental Protection Agency (USEPA) has proposed regulations on the selenium concentration in some wastewater discharge streams as recently as November 2019 (period for review closed in January 2020). For example, in these latest proposed regulations the primary selenium discharge limits in flue gas desulfurization (FGD) wastewater streams at coal-fired power plants are: 16.6 μg/L long-term average; 76 μg/L daily maximum limitation; and 31 μg/L monthly average limitation.
Thus far, EPA’s best available technology (BAT) for selenium removal has been biological treatment with adsorption of the oxidized selenium compounds on an organic substrate and subsequent digestion of the selenium oxides by microorganisms that convert the compounds to elemental selenium retained by the microbes. These systems are very large and expensive, and they require periodic removal of spent organisms and replenishment of the microbiological substrates.
A previous limiting factor of non-biological, chemical/physical selenium removal from wastewater streams has been the interference of competing ions with the oxidized selenium compounds in precipitation reactions. Water is the closest thing to a universal solvent, and virtually all wastewater streams contain numerous constituents. For example, power plant scrubber effluent typically has somewhat high concentrations of the major hardness cations, which along with sodium are counterbalanced by the anions chloride and sulfate.
All mining operations involve some disturbance of the earth’s crust, which releases natural selenium contained within, which is then captured by water. Selenium enters a refinery as a naturally occurring component of crude oil. During the refining process, selenium tracks along with sulfur species and can concentrate in both the sour water concentrators and sour water strippers.
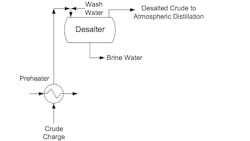
Desalting of crude oil prior to further treatment is a critical process at refineries (see Fig. 1). Desalting takes out most of the non-organic compounds and puts them in a stream whose treatment presents challenges. Selenium compounds are often part of these impurities.
A New Physical/Chemical Process
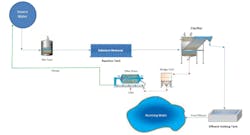
A proprietary and patent-pending process from ChemTreat called SeQuester™ offers a physical-chemical alternative to the biological method of selenium removal that can easily ramp up or down with changes in process flow to meet varying operational demand. ChemTreat utilizes a tailored formulation in the customers’ existing process equipment for a series of chemical reactions with the selenium. Lab tests and field results have shown that not only is selenium effectively removed but additional metals and metal oxides are also captured. These metals include arsenic, mercury, and molybdenum. The effluent from the SeQuester process, containing fine precipitates, is treated with a specialty flocculant in a clarifier. The solids drop out and are periodically blown down to the sludge holding tank.
The final step in the process is conversion of the sludge to a solid cake via standard sludge dewatering equipment. Data from Toxicity Characteristic Leaching Procedure (TCLP) tests have demonstrated that this cake is very stable and can be landfilled as non-hazardous waste. Filtrate from the press is routed back to an inlet equalization tank at the beginning of the process.
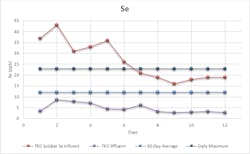
Because the power industry is one of the key targets for selenium removal, the initial pilot was performed at a large East Coast utility. Average influent total selenium was 230 ppb (54 percent as SeO3, 46 percent as SeO4), and average treated effluent after the process was 4.1 ppb Se (as SeO4).
As shown in Figure 3, only soluble influent selenium is reported because the insoluble portion is removed in upstream processes. The graph illustrates that the technology can control total selenium below the 30-day average limit. Representative samples of sludge from the sludge tank were collected and submitted for TCLP analysis, and this data showed that the selenium was tightly sequestered within solids and thereby rendered safe. The utility is now planning to install a full-scale system on a permanent basis.
But what about other industries? Besides the potential applications listed earlier, numerous other possibilities exist. For example, selenium is an impurity in some groundwaters or other makeup water sources to industrial plants. If these plants have cooling towers, wherein the water “cycles up” during standard operation, the selenium concentration in the blowdown may exceed the plant’s discharge limit.
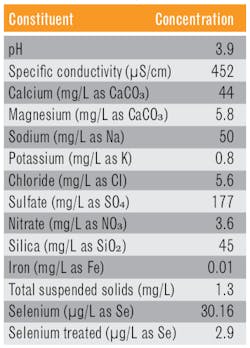
The mining industry is not facing a standard selenium discharge limit like the power industry, but rather selenium discharge is likely to be regulated in each facility’s NPDES permit. A State of Kentucky petition for withdrawal from the NPDES program3 includes a map that shows selenium levels from hundreds of samples in coal seams throughout the Appalachia region. Many samples show selenium concentrations in a low part-per-million range but where state-regulated NPDES discharge guidelines may be as low as 6 ppb. Table 2 illustrates the chemistry of an open pit mine water sample, and it shows the selenium reduction achieved on the sample with the SeQuester process.
The data from another bench-scale test outlines an example of selenium reduction potential from oil refinery wastewater: 24.4 μg/L from untreated wastewater; <2.5 μg/L from treated wastewater.
In many states (California, for example), groundwater is being regulated for selenium discharge, creating challenges for industrial facilities. Selenium is found in the upper Cretaceous and Tertiary marine and sedimentary deposits that form the California Coast Ranges and inland Central Valley basin. Sedimentary rocks, particularly shales, have the highest naturally occurring selenium content. The natural weathering of geologic strata containing selenium can lead to selenium leaching into groundwater and surface water.
Two major categories of anthropogenic activities are known to cause increased selenium mobilization and introduction into aquatic systems. The first is human disturbances to the geological sedimentary deposits; the second is irrigation of selenium-rich soils. Additional sources in this area include five oil refineries along San Francisco Bay.4
Bench-scale tests of this process on a groundwater sample indicated selenium concentrations of 11 μg/L on untreated cycled-up groundwater and 1.5 μg/L on treated cycled-up groundwater.
Ash Pond Remediation Potential
This technology offers great potential to assist with ash pond remediation at both operating and closed coal-fired power plants. Installation of permanent equipment might not be viable at some of these facilities, but a number of the leading water/wastewater treatment equipment firms offer mobile systems that could be brought in on a temporary basis to clean up the ponds.
Conclusion
For all of the above-mentioned applications and others, a critical feature about this new technology is that it utilizes standard equipment and thus can be incorporated at both existing and new facilities without the need for installation and operation of capital-intensive specialty systems. Certainly, much planning must go into any proposed application, but this process offers a potentially attractive alternative to biological methods for selenium removal from a variety of process and wastewaters. WT
Authors' Note: Results are examples only. They are not guaranteed. Actual results may vary.About the Authors
Vladimir Djukanovic is research and development project consultant with ChemTreat. He has fifteen years of R&D laboratory experience, thirteen years R&D industrial water treatment experience, and holds two U.S. patents. He has B.S. in chemistry from Virginia Commonwealth University. He may be reached at [email protected].
Brad Buecker is senior technical publicist with ChemTreat. He has many years of experience in or affiliated with the power industry, much of it in steam generation chemistry, water treatment, air quality control, and results engineering positions with City Water, Light & Power (Springfield, Ill.) and Kansas City Power & Light Company’s La Cygne, Ks., station. He also spent two years as acting water/wastewater supervisor at a chemical plant. Buecker has a B.S. in chemistry from Iowa State University with additional course work in fluid mechanics, energy and materials balances, and advanced inorganic chemistry.
Dave Karlovich is a U.S. Navy veteran with over 30 years of experience in water treatment applications supporting both the nuclear and fossil-fired power industries. His recent focus has been on chemistry to improve both the process side and wastewater treatment of wet flue gas desulfurization systems at plants throughout the country. He is co-author of ChemTreat’s patent-pending FGD1105 technology to improve SO2 removal and limestone reactivity in wet scrubbers.
References
1. Reeder, T.S., and J. Schneider. “Total Maximum Daily Loads and Site-Specific Objectives for Selenium in the Newport Bay Watershed, Orange County, California,” Draft Technical Staff Report for Peer Review, October 2009.
2. Reeder, 2009.
3. Petition for Withdrawal of the National Pollutant Discharge Elimination System Program, Delegation from the State of Kentucky, 2010. See: http://kftc.org/sites/default/files/docs/ky_npdes_petition.pdf
4. Reeder, 2009.