Supercritical water oxidation for the recovery of critical minerals and materials from wastewater sludge
Critical minerals and materials (CMMs) are those that have essential uses yet are in jeopardy regarding their domestic or global supply versus demand in the future. Many of these CMMs are found in wastewater sludge and are retained in the resulting biosolids, ash or residuals post sludge treatment. Contamination by hard-to-treat organics such as antibiotics, per- and polyfluoroalkyl substances (PFAS) and microplastics restrict the use of these CMM-rich products. Supercritical water oxidation (SCWO) is a treatment technology that produces residuals which are rich in CMMs yet free of organic contaminants. While the use of SCWO for CMM recovery is very much in its infancy, the scientific principles backing the practice suggest that more research efforts should be undertaken to promote CMM recovery from the SCWO treatment of wastewater sludge. This will help provide a robust and environmentally friendly supply of CMMs.
Resource recovery of CMMs from wastewater sludge
CMMs are those that are required in providing essential goods and/or services but are projected to be lower in supply than demand in the future. While certain agencies have a defined list of CMMs (e.g. U.S. Department of Energy), there is no universal list of CMMs. Geographically, CMMs are defined by the supply of a particular CMM within a region based on the capacity of the domestic reserves, cost and accessibility of imports, available synthesis methods and resource recovery capabilities. Additionally, every industry has unique demands for minerals and materials, which may render one mineral or material critical in a certain industry but not others.
The demand for CMMs is influenced by the development of new uses for CMMs, population growth or decline and changes in living standards1. To maintain "business as usual" or adapt to growing demands, it is pertinent to responsibly source CMMs. In many countries, the supply of many CMMs relies on domestic and foreign mining of finite reserves, which is not sustainable and contributes to environmental pollution. An alternative to mining is the synthesis of CMMs, which is currently limited to a few CMMs and for some CMMs may not be feasible. The third alternative is using resource recovery to recycle CMMs found in “waste products” such as wastewater sludge.
CMMs found in sludge
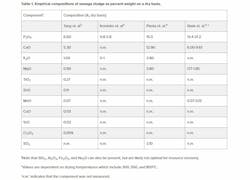
Wastewater sludge is a reservoir for CMMs that enter the wastewater treatment plant (WWTP) from municipal, commercial and/or industrial wastewater flows. The composition of CMMs from commercial and industrial dischargers relies heavily on the industries they belong to, and quantitative data on which CMMs are in various commercial and industrial wastes is sparse. Therefore this article focuses on municipal sludge for which there is adequate peer reviewed literature. CMMs from municipal sources largely originate from the excretion of excess CMMs from food into urine and/or feces. Through wastewater treatment, these CMMs concentrate in the sludge, to proportions that may be viable for recovery. Table 1 illustrates select components in wastewater sludge that are potential CMMs, depending on the geographic area and industry of interest. Some commonly measured and/or significant components, such as SiO2, are not reported as resource recovery is not likely to be competitive to current sourcing methods.
A primary example of how the resource recovery of CMMs in sludge can be used to alleviate supply versus demand issues is that of phosphorus. Firstly, Table 1 illustrates that phosphorus (as P2O5) may be viable as it can account for a large fraction of the sludge by dry weight (up to 21.2%). In fact, it is estimated that there is enough phosphorus from human waste streams to supply 22% of the global phosphorus demand6. A now common practice is to land apply sludge (or processed sludge)7 as fertilizer for agricultural purposes. This practice is in jeopardy, as there are mounting concerns over PFAS and/or microplastics, among other contaminants. Currently, Maine stands as the first state that has prohibited the land application of sludge and biosolids8. Other states, such as New York, are in the process of enacting legislation to mandate more rigorous testing and treatment before employing biosolids for land applications.
This article will discuss how SCWO can address these restrictions by producing residuals rich in CMMs yet free of organic contaminants, for safe reuse.
Basics of supercritical water oxidation (SCWO)
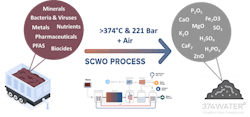
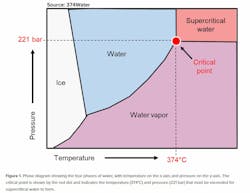
Supercritical water oxidation is a next generation technology that transforms organic wastes into carbon dioxide, clean water and inorganic salts (i.e. minerals) by leveraging the properties of supercritical water (SCW). In addition to water, ice and water vapor, water has a fourth phase — supercritical. Figure 1 illustrates a phase diagram with pressure plotted against temperature. SCW forms when water is heated (>374°C) and pressurized (>221 bar) above the critical point (shown by the red dot). SCW has properties that are nearly opposite of those in water at ambient temperature and pressure (20°C and 1 bar). In SCW all organics (i.e. carbon containing materials and compounds) become soluble and dissolve, forming a single SCW-organic phase. On the other hand, inorganics (such as minerals) precipitate. These properties enable the powerful transformation of organic wastes and potential recovery of inorganics.
SCWO systems operate by heating and pressurizing a mixture of water and organic waste above the critical point (>374°C, >221 bar). The water in the feed becomes supercritical and completely dissolves all organics and oxygen in the feed, forming a single water/organic/oxygen phase. In this single phase, oxidation reactions proceed rapidly and to completion, destroying all organics. Inorganics already present in the feed pass through the system but may be further oxidized.
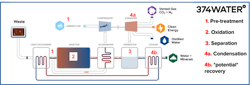
374Water has made substantial improvements to SCWO, with the EPA PITT team labeling 374Water’s SCWO system as the “third-generation of SCWO.” 374Water’s patented technology (US-11420891-B2) boasts improved safety, enhanced corrosion control, efficient energy usage and a prefabricated, modular and compact design. Enhancements to the system boost the suitability of treatment for a plethora of waste streams. Figure 2 illustrates 374Water’s AirSCWO system.
Firstly, the feed (e.g. sludge) is preheated and compressed ambient air is mixed in. The addition of ambient air improves safety (as compared to O2 or hydrogen peroxide addition) and improves oxidation efficiency in the reactor. The feed is then sent to the reactor where it is heated above 374°C and pressurized above 221 bar. The water in the feed becomes supercritical and organics completely break down via oxidation reactions in under one minute. The resulting stream contains supercritical water, dissolved gases and inorganics. This stream is cooled and the water (now subcritical) is separated from the gas. The wet gas is sent to an expander where gas, containing mostly CO2 and N2, is separated from water vapor, producing a distilled water stream. The expander recaptures the energy of the compressed gas and produces electricity. The liquid effluent from the phase separator, which contains dissolved and precipitated minerals, is available for use. When dried, this stream produces a CMM containing ash-like material herein called "residual." This article will describe the potential of recovery of CMMs from these residuals.
CMMs in SCWO residuals
While the composition of the sludge input to the SCWO reactor varies greatly, the SCWO residual has been shown to contain common components which are consistent with the large fractions of these compounds in the sludge feed itself (Table 1). Table 2 illustrates the empirical percentages of these and other CMMs in the SCWO residuals found in the literature (from a non-exhaustive search). Note that not all components listed in Table 1 are mirrored in Table 2, as they were not measured.
In some instances, the desired CMM to be recovered is a product of SCWO, not an input. The destruction of organics or conversion of inorganics through SCWO produces valuable CMMs that may not have originated in the waste itself. Table 3 provides a few examples of how compounds contributing to the COD, PFAS, biocides, bacteria and heavy metals can be converted into value-added CMMs. These examples illustrate how SCWO can serve a dual purpose of producing materials for recovery and destroying harmful contaminants.
As can be seen in Table 3, the conversion of these contaminants into useful end products occurs with minimal inputs. Each reaction (apart from phosphoric acid) is further enhanced by the addition of O2, which can be safely added through the use of ambient air. The destruction of halogenated compounds, including PFOA and triclosan, is aided through the addition of an alkali to prevent the formation of highly corrosive HX compounds, where X is the halogen. Potential benefits of the conversion of contaminants to CMMs warrant further research efforts to both characterize the end products and develop extraction techniques to recover these products.
Applications of SCWO for mineral recovery
Table 4 presents common uses of these CMMs and which processes resource recovery would replace in sourcing these CMMs. It is important to note that while there is the potential to use SCWO for sourcing these CMMs, the resulting purity (and economic considerations for obtaining the necessary purity for industrial applications) may render SCWO a technology to supplement, rather than replace, current sourcing methods.
Engineering implications and outlook
One of the main factors influencing the engineering implications of SCWO for CMM recovery from sludge is that the composition of sludge is inconsistent12 between different WWTPs. While there is no one size fits all SCWO reactor conditions to treat sludge for CMM recovery, the conditions can be easily modified and tested through bench scale treatability studies13. Additionally, variations in sludge within a WWTP (e.g. between primary and secondary sludge) may be resolved by blending different sludges, even across multiple days, to achieve certain CMM concentrations, sludge volumes, calorific values and % solids required for optimal SCWO treatment and recovery. Finally, if CMM recovery is to be optimized, the initial amount of building block or CMM in the feed should be quantified first (e.g. total P, metals, PFAS, etc.).
Another major consideration when recovering these minerals and materials from the SCWO product is the purity required for the final application(s). This may lead to the conclusion that additional refining post-SCWO is necessary for resale to markets with high environmental importance and/or economic value. To inform the impact of this, lifecycle assessments should be undertaken to understand the environmental impact of recovering CMMs from SCWO versus traditional sourcing methods. In addition, an assessment of the supply-demand in the near and long-term should be considered to prioritize which CMMs SCWO should be optimized for.
The production of CMMs from SCWO is promising on a fundamental scientific basis and in few aforementioned studies. To date, from a public data availability perspective, there are no companies that refine and sell CMMs from SCWO residue. The progress of which may be hindered by a potential lack of overlap in the skill force needed to design and optimize reactors versus the CMM extraction system. It is suggested that the recovery of CMMs is developed in parallel to the development and optimization of SCWO reactors. These efforts will help promote a circular economy, improve the environmental impact of CMM sourcing, and alleviate the current supply-demand issues facing CMM production and use.
Naomi Lynn Senehi, an Engineer and Global Access Lead at 374Water, holds a Bachelor's and Master's in Environmental Engineering. With 5 years of R&D experience, she specializes in developing Supercritical Water Oxidation application solutions to diverse waste streams.
References
1. Kesler, S. E.Proceedings for a workshop on deposit modeling, mineral resource assessment, and their role in sustainable development. US Geological Survey. 55-62.
2. Tang, S., Zheng, C. & Zhang, Z. Effect of inherent minerals on sewage sludge pyrolysis: Product characteristics, kinetics and thermodynamics. Waste Management 80, 175-185 (2018).
3. Kominko, H., Gorazda, K. & Wzorek, Z. The possibility of organo-mineral fertilizer production from sewage sludge. Waste and Biomass Valorization 8, 1781-1791 (2017).
4. Piasta, W. & Lukawska, M. The effect of sewage sludge ash on properties of cement composites. Procedia engineering 161, 1018-1024 (2016).
5. Stark, K., Plaza, E. & Hultman, B. Phosphorus release from ash, dried sludge and sludge residue from supercritical water oxidation by acid or base. Chemosphere 62, 827-832, https://doi.org/10.1016/j.chemosphere.2005.04.069 (2006).
6. Mihelcic, J. R., Fry, L. M. & Shaw, R. Global potential of phosphorus recovery from human urine and feces. Chemosphere 84, 832-839, doi:10.1016/j.chemosphere.2011.02.046 (2011).
7. Division, M. D. o. E. Q. W. R. What are biosolids, how are they used,and are they safe? (2014).
8. Hughes, S. G. PFAS in Biosolids: A Review of State Efforts & Opportunities for Action. ECOS (2023).
9. Stendahl, K. & Jäfverström, S. Phosphate recovery from sewage sludge in combination with supercritical water oxidation. Water Science and Technology 48, 185-190 (2003).
10. Stendahl, K. & Jäfverström, S. Recycling of sludge with the Aqua Reci process. Water Science and Technology 49, 233-240 (2004).
11. Stark, K. Phosphorus Release and Recovery from Treated Sewage Sludge. KTH Architecture and the Built Environment (2005).
12. Sichler, T. C. et al. Variation of the element composition of municipal sewage sludges in the context of new regulations on phosphorus recovery in Germany. Environmental Sciences Europe 34, 84, doi:10.1186/s12302-022-00658-4 (2022).
13. Pérez-Elvira, S., Nieto Diez, P. & Fdz-Polanco, F. Sludge minimisation technologies. Reviews in Environmental Science and Bio/Technology 5, 375-398 (2006).