Activated carbon has more than 2,500 commercial product applications. Most wastewater plants use the carbons to purify the water and air leaving a facility. However, you will not find their characteristics and properties covered in "formal" education. You learn about them on the job.
Activated carbon is an inert solid adsorbent material commonly used to remove diverse, dissolved contaminants from water and process gas-phase streams. It is made from almost any feedstock that contains carbon, including coconut shells and coal family members, as many readers will already know.
Adsorption is the accumulation of a gas or liquid on the surface of a liquid or solid substrate, as opposed to absorption, in which the encroaching substance enters the substrate’s bulk or volume.
Activated carbon is porous, inexpensive and readily available for use as adsorbents, furnishing a large surface area to remove contaminants. It has more useful surface area per gram than any other material available for physical adsorption. In fact, a teaspoon of activated carbon has more surface area than a football field.
Physical phenomena
Because of its rare characteristics, activated carbon possesses an exceptional ability to capture water-dissolved contaminants that include taste-, odor-, color- and toxic-promoting species. Removal takes place through adsorption phenomena based on surface interactions between contaminants and carbon graphitic platelet surfaces.
These contaminant-carbon surface interactions occur through Van der Waal forces and induced dipole interactions. Activated carbon graphitic platelets induce neutral organic molecules into intra-molecular dipoles. The induced dipoles cause the molecules to be attracted to each other and stick together, so they precipite out of solution in the carbon’s nano-sized pores or adsorption spaces. This is referred to as premature condensation, facilitated by the activated carbon.
Figure 1. These representations show activated carbon made from wood, coconut shells and bituminous coal. All graphics courtesy of PACS Activated Carbon.
Activated carbon manufacturers use different feedstocks and process parameters to make a variety of pore size distributions available. Proper pore structure selection is essential to solve aqueous- and gaseous-phase problems with activated carbon.
Figure 1 shows realistic representations of activated carbons manufactured from wood, coconut shells and bituminous coal. These carbon types are sold and used in different forms: powder, granular, pellets, blocks and composites. The difference is seen in the size of the graphitic platelets represented by the heavy black lines and how close they are together, as indicated in the figure.
Powdered activated carbon
Powdered, micron-sized activated carbon particles are milled from millimeter granular activated carbon and exhibit faster kinetics and a greater capacity for contaminant removal, when compared to carbons with larger particle sizes.
Powdered activated carbon can be used for sporadic contaminant episodes, such as algae blooms and industrial spills, that contaminate municipal influent waters. Powder can be added to the clarification process settling unit to remove these contaminants with activated carbon. It can also protect fixed activated granular carbon beds against sudden influent contamination.
Plants can use powder instead if they lack the infrastructure to use granular activated carbon or do not have enough granular carbon between the influent and the effluent to economically use for removal in sporadic contaminant episodes. The single-use powdered activated carbon is used as a batch process to remove contaminants to acceptable regulated maximum contamination levels (MCLs) but not necessarily to zero or non-detected contamination.
Granular activated carbon
Millimeter-sized granular activated carbon can remove contaminants to concentrations below analytical detection limits, and compared to powder, it requires only about one-fourth the amount of carbon between influent and effluent.
However, a plant needs proper infrastructure to install the fresh carbon and remove the spent granular activated carbon for furnace reactivation. Reactivated activated carbon costs about half as much as fresh or unused granular activated carbon. Granular activated carbon use is a continuous process, and it is a multiple-use product based on thermal reactivation. Thermal reactivation enables the carbon to be classified as "green chemistry."
Where the possibility of industrial pollution is relatively high, more activated carbon must be readily available for possible emergencies. It can be kept in fixed vessels between the influent and effluent, and more powdered carbon is needed as well.
Finally, pellets, or extra-large carbon granules, are used to control vapor phase municipal wastewater hydrogen sulfide and other odors. These relatively large forms of activated carbon enable gas streams to flow through carbon beds uninhibited. This decreases the use of fans and energy necessary to blow gas streams through tight beds. Regular and catalytic carbons are used for hydrogen sulfide odor control.
With regular carbon, mobile hydrogen sulfide is oxidized to immobilized sulfur, which accumulates on the carbon surface. Using elemental sulfur build-up on working carbon has determined when the carbon needs to be replaced with fresh carbon at laboratories. Catalytic carbons transform hydrogen sulfide to sulfuric acid by oxidation. Sulfuric acid on this catalytic carbon can be washed from used carbon with water and be reused on site many times.
Figure 2. A horizontal bed configuration looks like this, whereas vertical beds take advantage of gravity flow.
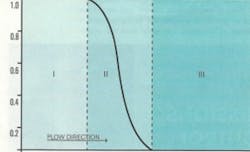
Mass transfer zone
Aqueous and gas phase applications develop a moving contaminant mass transport zone (MTZ) as more contaminated water or gas passes through a bed. Carbon beds are usually 3 to 10 feet deep and composed of stratified activated carbon, where smaller sized particles are on top of a working carbon bed and the largest sized particles are on the bottom.
Do not mix used and unused carbon in a process. The MTZ illustrated in Figure 2 has a horizontal bed configuration, but beds are typically vertical to take advantage of gravity flow. Bed stratification must be maintained after backwashing to remove solids that may accumulate on top of the bed.
Activated carbon removes water soluble organics and solids from water by backwashing. This MTZ has three working zones:
- Zone 1 (between A and B, a portion of the total length of the carbon bed) is completely used and no longer removes water-soluble contaminants.
- Zone 2 (between B and C) removes variable amounts of contaminants. The shape of this curve will reflect the concentration profile of the contaminants exiting the carbon bed at breakthrough. Water applications typically have much longer MTZ distance between B and C than gas-phase applications, which typically have much smaller MTZs. The MTZ shape may be sharp or broad depending on how strongly the carbon adsorbs the adsorbates.
- Zone 3 (between C and D) is unused activated carbon. With increasing bed service-time and exposure to contaminants, the distance between A and B increases and between C and D decreases. MTZ distance from B to C is constant.
Maximizing performance
For improved performance and economics, the typical configuration in operation of multiple beds of activated carbon is a sequential series. Multiple beds in a series enable complete carbon bed use through breakthrough, where influent and effluent are equivalent in contaminant concentrations. This is because in operation any remaining backup beds in the series start another MTZ as needed.
This lead-and-lag bed configuration enables treatment of a maximum number of gallons of water per pound of activated carbon before spent carbon must be replaced with fresh.
The working goal is high-quality potable water at the lowest cost. The final beds of activated carbon in the sequential series complete the polishing to remove trace contaminants, and to provide safe, quality drinking water. By changing out the earlier completely exhausted carbon beds with fresh carbon (when bed influent and effluent concentrations are equivalent), the later beds function longer as the final polisher and provide a safety margin.
When samples are taken to profile a carbon bed, they should be taken from top, middle and bottom. This type sampling allows more accurate estimate in locating the MTZ and remaining service time for the carbon bed.
Spent activated carbon
Activated carbon does not last forever. It needs a periodic change-out with fresh virgin or reactivated carbon. Pores or physical adsorption spaces, which are nanometer-sized volumes between the graphitic platelets, eventually fill and are no longer capable of removing adsorbates. Carbon pores are heterogeneous and vary in adsorption energy from strong to weak. Note the graphitic platelet spacing in Figure 1. Carbon graphitic platelets that are close together provide high adsorption potential energies, and wide platelet spacings have relatively low adsorption energies.
Drinking water plants have two main choices for change-outs: purchase virgin or unused carbon or use reactivated carbon. Following several reactivation cycles, the efficacy of reactivated carbon will diminish and must be replaced by fresh, virgin carbon.
Sometimes widening the pore size distribution with reactivation is beneficial, especially for larger molecules and higher molecular weight adsorbates. However, water soluble, low molecular weight compounds at trace concentrations such as trihalomethanes may not be as readily adsorbed and could develop a longer MTZ when used with reactivated carbon with a wider pore size distribution.
Further discussion of this topic will cover test methods to aid water plant personnel to select the best activated carbon for a given application and to monitor the efficacy and life cycle of the carbon through its final disposal.
Henry Nowicki, Ph.D. and MBA, is president and senior scientist for PACS Activated Carbon Services. He may be reached at [email protected], 724-457-6576 or pacslabs.com. George Nowicki is the PACS laboratory director. He may be reached at [email protected]. Wayne Schuliger, P.E., teaches a short course on design, operation and troubleshooting of activated carbon adsorbers.
PACS Activated Carbon Services hosts the International Activated Carbon Conference and training courses on carbon technology biannually in February in Orlando and in September in Pittsburgh.