The microbial oxidation of reduced sulfur species is a well-studied field. Sulfur oxidation is an ancient metabolic process, and sulfur oxidizing bacteria are an ecologically and taxonomically diverse group of bacteria with varied abilities for utilizing reduced sulfur compounds as substrates. Different species use different enzymes, pathways, and mechanisms of electron transport and energy conservation for the oxidation of a given substrate, but all gain energy from capturing electrons from the oxidation of sulfide to sulfate.
The general metabolic pathways of the Thiobacilli group of bacteria, however, are all similar. These bacteria use a metabolic pathway that can both produce and consume elemental sulfur as an intermediate product during the complete oxidation of sulfides to produce sulfate.1 Sulfur can be used for energy storage by these bacteria when oxygen limits the conversion of sulfide to sulfate. Thiobacilli species gain some energy during the conversion to sulfur, but when sulfide is no longer available, they can switch to using the sulfur as an energy source. The oxidation of sulfide to sulfur utilizes only one-fourth the amount of oxygen that oxidation of sulfur to sulfate requires, as shown in equations 2, 3 and 4.
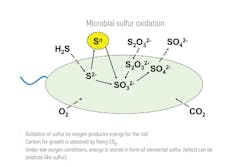
These bacteria can, alternatively, use sulfide, sulfur or thiosulfate as the energy source while producing sulfate as the end-product because they fix carbon dioxide to make their biomass in a manner similar to the process in plants. Figure 1 represents sulfur oxidation in sulfur-oxidizing bacteria. Some species of Thiobacilli operate best under acidic conditions while others operate better under neutral-to-slightly acidic conditions. To avoid issues with corrosion, the cultures that perform best under neutral-to-slightly acidic conditions will be used in the treatment of sour water.
The Thiobacilli bacteria have evolved their enzymes for metabolizing sulfur species over millennia – and to compete with one another. The rate of sulfur oxidation by these enzymatic pathways is orders of magnitude more efficient than abiotic oxidation of sulfide by molecular oxygen. Therefore, microbial metabolism is the major mechanism by which reduced forms of sulfur are oxidized in the natural environment. Various groups have made use of these capabilities in many commercial processes including leaching of metals in mining applications and for removing sulfide from vent gas streams. Biological sulfide oxidation has been used to treat produced water in the oil field, in the past by using crude biological processes.
Microbial oxidation for sulfide removal
The biological removal of sulfides has many advantages over physical/chemical treatment methods. These treatment methods include:
- Air stripping: Sulfide is highly volatile, and at slightly acidic pH, it tends to partition out of the aqueous phase into the vapor phase, and, therefore, air stripping will remove sulfur from the aqueous phase. However, this process becomes less efficient as the concentration of sulfide in the aqueous phase depletes. Also, the sulfide is not destroyed. Rather it proportions into the stripped air. This sulfide still needs to be captured with an adsorbent or incinerated. Both are problematic. The adsorbent adds cost including the cost of handling and disposing of large volumes of spent adsorbent, which may be a hazardous waste (if caustic). Incineration produces acid sulfur gases, which also need scrubbing and may cause corrosion issues due to acid formation.
- Chemical oxidation: Many oxidants can be added to the sour water stream to oxidize sulfide. The problem with most of these oxides is they generally produce elemental sulfur as the end product of the oxidation at slightly acidic-to-neutral pH values. To produce sulfate, the pH needs to be raised to greater than pH 9.0, which adds considerable chemical costs. Also, the amount of oxidant required to produce sulfate, rather than sulfur, is about four times greater, which adds considerable cost.
- Chemical precipitation: The addition of iron salts will produce insoluble iron sulfides that can be separated by clarification. The problem with this approach is the high material-handling costs and amount of sludge produced. The amount of labor to operate the dewatering equipment also will be considerably higher than that required for the bioreactors. The iron sulfide sludge becomes pyrophoric when dry, so special care needs to be taken when handling this material and in its disposal. Due to its pyrophoric nature, it is generally regarded as a hazardous material.
Biological oxidation of sulfide, in which oxygen in the air is the oxidant, represents a much more efficient approach. This is a widely available and inexpensive source of oxidant.
Although sulfur may be an intermediate in the conversion of sulfide to sulfate, it will not be present in the final effluent and does not need to be recovered or disposed of. Excess sulfur can be digested by bacterial oxidation and will provide a food source during extended downtimes.
Process for sour water treatment
One process for sour water treatment balances many competing processes to provide an efficient and economical solution for the removal of hydrogen sulfide from sour water and conversion of the reduced sulfur species, including thiosulfate, to sulfate. This conversion process must produce an effluent water quality that is suitable for reuse or discharge at point of use.
Thus, the treatment process must balance a number of competing goals: minimizing the stripping of hydrogen sulfide from the aqueous phase to the air phase, maintaining a neutral-range pH in the final effluent and producing a clarified effluent of less than 20 parts per million (ppm) of total suspended solids and a zero ppm concentration of elemental sulfur solids.
The approach taken for this particular design is to combine three proven biological processes into a single integrated treatment process. These processes include biofiltration of sulfide present in the bioreactor vent gas, conversion of sulfide and thiosulfate to elemental sulfur and sulfate within the bioreactors, and the digestion of elemental sulfur recovered from the bioreactors into sulfate. The integration of these processes will allow a balance between competing requirements of producing no sulfide emissions into the atmosphere and a neutral pH of the final effluent from the treatment plant with no sulfur present.
Figure 2. The sulfide profile of a pilot system treating sour water
The biological oxidation of sulfides and sulfur-containing compounds is a widely practiced industrial process. The use of sulfur oxidizing bacteria to treat hydrogen sulfide present in air exhaust or vent air streams is a commercial process practiced by a number of companies.2 It might seem obvious that a simple solution would be to strip all the sulfide out of the aqueous phase into the biofilter treatment system. The problem here is that, for biofilters to function properly, there must be a large excess of air compared to the concentration of sulfides in the filter. The amount of air required and the size of the biofiltration vessels would be prohibitively large, so the most effective design aims to minimize the amount of sulfide stripped from the aqueous phase into the biofiltration system.
The biofiltration system does, however, have some significant advantages over other methods for removing the stripped sulfide from the vent gas. The sulfide is converted to sulfate due to the excess of oxygen to sulfide. No adsorbent must be replaced nor spent material to be disposed of. The sulfate produced from sulfide is present in the effluent from the trickle bed and can be discharged to the bioreactor effluent. The biofiltration system in the UOP design will treat about 15 percent of the overall sulfide present in groundwater that will be stripped from the bioreactors.
The biological oxidation of dissolved and particulate sulfides is widely practiced in the wastewater treatment and mining industries. The problem with biological oxidation is that the bacteria need a large amount of oxygen to oxidize the sulfides all the way to sulfate. The volatility of sulfide is quite high, so high aeration results in considerable stripping of the sulfide. Therefore, to balance stripping with oxidation, lower aeration is necessary. Under lower aeration, the sulfide will be oxidized to elemental sulfur rather than sulfide.
The goal of the process described is not to produce sulfur but to convert any sulfur formed to soluble sulfate. The sulfur formed in the leading stage of the system is removed in two ways. Some of the sulfur is retained in the packed bed, but some is entrained in the water flow. The sulfur that is suspended in the flow is oxidized to sulfate in the lagging bioreactor sections where higher rates of aeration are applied to complete the oxidation of elemental sulfur to sulfate ions. Elemental sulfur is not volatile, so high aeration can be applied with no issues with sulfur volatilization. The goal of this section is to ensure that little sulfur exits the final bioreactor section. This effluent can be easily filtered to ensure zero sulfur solids in the final effluent. This design allows a relatively small bioreactor system with a six-hour total retention time to reduce sulfide from up to 300 ppm to less than 0.2 ppm in the final effluent.
This does, however, leave a large amount of sulfur solids retained in the leading bioreactors. To deal with the entrapped elemental sulfur, bioreactors will be operated in parallel. As one bioreactor is treating the sulfide-laden water, the second bioreactor will be operated in batch mode to ensure complete conversion of entrapped sulfur-to-sulfate ions.
Figure 2 illustrates the performance of a pilot system treating sour water at a client’s site. The effluent sulfide level was predominately less than 0.2 ppm. The formation and removal of elemental sulfur formed in the bioreactor is illustrated in Image 1. This shows the formation of sulfur solids and their removal as the water transfers from the leading chambers of the bioreactor to the latter chambers.
References
- Cornelius G. Friedrich, Dagmar Rother, Frank Bardischewsky, Armin Quentmeier and Jörg Fischer. “Oxidation of Reduced Inorganic Sulfur Compounds by Bacteria: Emergence of a Common Mechanism?” Appl. Environ. Microbiol. 2001, vol. 67, Vol. 7, 2873-2882.
- G. Soreanu, P. Falletta and M. Béland. “Removal of hydrogen sulfide from gas streams using biological processes – A review.” M. Syed, Canadian Biosystems Engineering. 2006, vol. 48, 2.1 – 2.14.
Steve Lupton, Ph.D., specializes in the application of bioprocessing to chemical and materials applications. Most recently, he brought his expertise to the design and evaluation of technologies for biomass treatment and utilization in support of the UOP Renewables Research Group, focusing on the conversion of algal biomass to fuels using catalytic liquefaction. Lupton was responsible for the development of Honeywell’s XCeed system, a fixed-film-based bioreactor system for environmental and fermentation processes.