Chemical and physical boiler feedwater treatment technologies are essential and readily available to provide high-quality water to minimize problems and achieve greater efficiency and productivity. Boilers are essential hot water and steam producers in many industries, including power generation plants. Hot water and steam production using feedwater that contains dissolved hardness ions, salts, oxygen and carbon dioxide cause chemical corrosion and scale formation that ultimately reduces the thermal efficiency and damages the boiler, decreasing the equipment’s useful life.
Literature on the topic of boiler feedwater treatment is substantial and readily available. This column provides an overview of several pretreatment technologies and water-quality factors that contribute to optimal boiler performance and efficiency.
Water and heat
Water has a higher heat capacity than most other common substances. In metric units, 1 small calorie of heat will raise the temperature of 1 gram of water 1oC. One kilocalorie of heat will raise the temperature of 1 liter of water by 1oC. The kilocalorie is used for measuring food calories. The heat of vaporization to convert water at 100oC to steam is 540 calories/gram.
In English units, a British thermal unit (BTU) is the amount of energy required to raise the temperature of 1 pound of water by 1oF at standard atmospheric pressure. One pound of water will require 970 BTUs to convert to steam at 212oF at 1 atmosphere pressure. One BTU is approximately equal to 252 small calories. The boiling point of water increases with increasing pressure and reduces at lower pressures.
Problem water components
Generally, with some exceptions, the Northeast, South and Northwestern U.S. have relatively soft natural water defined as having less than 60 milligrams per liter (mg/l) as calcium carbonate. The water in the geography between these areas tends to range from hard to very hard water, defined as 121 to 250 mg/l calcium carbonate). Groundwater sources commonly have more dissolved minerals and hardness, but there are surface waters, such as the Great Lakes and major rivers that have substantial mineralization. Surface water sources generally have higher total organic carbon (TOC) levels and higher suspended particles (turbidity). Virtually all surface municipal water supplies are filtered. Some municipalities soften their water with lime or lime soda treatment. However, each facility must determine the level of treatment necessary to provide acceptable boiler feedwater based on the equipment’s design and operating conditions.
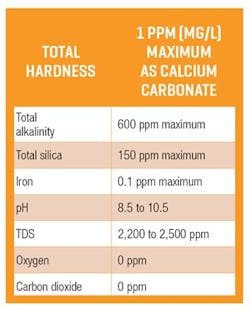
Rainwater has small amounts of dissolved solids and is often acidic and aggressive to metals. Other natural waters can have small to very large concentrations of dissolved mineral salts or total dissolved solids (TDS), TOC, suspended particles, gases (oxygen, carbon dioxide, nitrogen), plus pH (acidic, neutral, basic), alkalinity (acid neutralizing capacity), and hardness (calcium and magnesium and some other bicarbonates). Except for dissolved nitrogen, these impurities can significantly impact boiler life and performance. As a result, pretreatments are required to provide water that will not damage the system or reduce the boiler’s efficiency. Some suggested water quality factors to be considered in producing appropriate boiler water are listed in Table 1.
See the American Society of Mechanical Engineers (ASME), cited in the references, for much more on ASME guidelines for water quality and other aspects of watertube boiler operations. It includes aspects related to drum pressures, copper, iron, specific conductance, silica, hardness and alkalinity.
Water technologies for boiler feedwater treatment
The full range of standard water treatment technologies are available to pretreat boiler feedwater. The process choices and configurations depend on the composition of the source water, contaminants to be removed and costs.
Filtration and coagulation
Suspended particles may be organic or inorganic materials that did not dissolve in the source water. However, some particles might dissolve or precipitate in the heated water and cause problems. Particulate removal processes include conventional coagulation, flocculation, granular media and cartridge filtration. Coagulants, including iron and aluminum salts and designed organic polymers, can be used to facilitate particle sedimentation and filtration.
Softening
Hard waters contain calcium, magnesium and other metal bicarbonates that will be thermally converted to insoluble carbonates in the boiler while producing carbon dioxide. Hardness is commonly described in calcium carbonate equivalents, which incorporate the other contributing ions in the calculation. Conventional water softening processes including lime and lime soda, and cation exchange resins can significantly reduce the hardness ions. By separating most of the hardness ions during pretreatment, later precipitates of insoluble sludges and scale are significantly minimized.
Membranes
Membranes may also be used, possibly microfiltration or ultrafiltration. Membranes are identified by their respective pore sizes and the sizes and types of water components that they remove. They even remove various microbial contaminants based upon size exclusion. Microfiltration (MF), which removes contaminants of 0.1 to 1 microns (μm), is used as an alternative to conventional coagulation, flocculation, sedimentation and granular media filtration.
Controlling the composition of boiler feedwater and the water in a boiler is essential to successful and efficient operations. The available treatment technologies are based on the fundamental chemical and physical properties of the components being managed, and those standard water treatment methods and their selection are tailored to the specific problems to be addressed.
Ultrafiltration, (UF), with pore sizes of 0.001 to 0.1 μm, removes high-molecular-weight organic chemicals, including natural humics. However, excessive fouling is common unless the water has been pretreated.
Nanofiltration (NF), with pore sizes of ~0.001 μm, requires pretreatment to reduce fouling, but it can remove substantial amounts of multivalent inorganic ions as well as high molecular weight organic chemicals.
Reverse osmosis (RO), with pore sizes of 0.0001 to 0.001 μm, can remove more than 99 percent of most dissolved ions and many organic chemicals with molecular weights as low as a few hundred Daltons. Some performance exceptions include borates and arsenites/arsenates. RO treated waters will have a lowered pH, be more aggressive to metals and will probably need to be stabilized. RO will require pretreatment to reduce membrane fouling, possibly by MF or UF. Its ion removal capability is reflected in its use in the desalination of sea water and brackish water. That capability indicates that RO may provide the opportunity to use brackish groundwater that are frequently available in local geology. The fouling of membranes requires regular and perhaps frequent cleaning because of the pressure drop that will occur without this maintenance.
Internal boiler feedwater treatments
Other approaches that complement pretreatment involve internal treatment where chemicals are added to the water to prevent adverse consequences in the boiler. They are intended to reduce scale deposition on heat transfer surfaces in the boiler. Calcium and magnesium hardness can be solubilized or suspended by chelates and polymers that prevent scale and sediment formation. Phosphates precipitate several components so they can be removed in the accumulated blowdown, which is concentrated solids that are periodically drained from the boiler. Sulfates, silicates and phosphates form insoluble calcium and magnesium salts that must be managed.
Corrosion management
Several contributors to corrosion include oxygen and carbon dioxide gases, pH effects and certain salts. Corrosion is accelerated at high operating temperatures and low pH levels. Metals tend to be oxidized, for example, iron to iron oxides, by oxygen in the water. Carbon dioxide forms carbonic acid. Oxygen scavengers include reducing agents like sulfite, bisulfite, hydroxylamines and organic chemicals, such as erythorbates, which are isomers of vitamin C. Carbon dioxide can be removed by degassing.
Magnetic and other physical devices
Many catalytic, magnetic and electromagnetic devices have claimed to treat water to alter the hardness and otherwise improve other water quality characteristics. The Water Quality Association convened a science and engineering task force to examine published literature describing the performance of these technologies. It identified 34 out of 106 papers to be worthy of further review.
The results were mixed and frequently inconsistent, and experimental designs were often questionable. The review generally concluded that some reports were positive, but that objective testing standards should be developed and applied to determine whether performance claims are verifiable.
The Canadian Water Quality Association issued a position statement concluding that it “knows of no generally recognized scientific or technical evidence which proves that magnetic, electromagnetic or catalytic devices sold to treat water have any measurable physical or chemical effect of water quality.” It maintains that all product performance and benefit claims should be based on factual data obtained from tests, conducted by competent personnel, following established test procedures.
Conclusion
Controlling the composition of boiler feedwater and the water in a boiler is essential to successful and efficient operations. The available treatment technologies are based on the fundamental chemical and physical properties of the components being managed, and those standard water treatment methods and their selection are tailored to the specific problems to be addressed.
Water quality problems are universal, so the arsenal of available treatment methods is also widely applied. Possible alternative boiler feedwater sources are recycled municipal wastewaters, industrial process waters and steam condensate, and desalinated brackish waters. They provide opportunities to reduce purchased and total water consumption, withdrawals and wastewater discharges as well as to fine-tune the treated water’s composition. The problems are understood and the available information resources are substantial.
Substantial debate continues surrounding the capabilities of magnetic, electromagnetic and catalytic devices marketed as water conditioning systems. This column provided a limited overview to assist users who are obtaining more detailed information and support.
References
- ASME. http://www.pdhonline.com/courses/m165/m165content.pdf. Accessed May 2017.
- Conner, S. & Bloom, D.. Essentials for a sound boiler water treatment program-2.pdf. Accessed May 2017.
- WQA Magnetics Task Force Report, March 2001. https://www.wqa.org/Portals/0/Technical/Technical%20Fact%20Sheets/MagneticsTF_Rpt.pdf. Acessed May 2017.
- CWQA Position Statement, March 1987. http://www.ilovemywater.com/UserFiles/Documents/What%20Is%20CWQA.pdf. Accessed May 2017.
- Boiler Feedwater Treatment Part I and Part II. http://www.sedifilt.com/technical_library/boiler_feedwater_treatment_fundamentals. Accessed May 2017.
- Boiler Corrosion Inhibitors. http://www.chemtreat.com/solutions/boiler-corrosion-inhibitors. Accessed May 2017.
Joseph Cotruvo, Ph.D., BCES, is president of Joseph Cotruvo and Associates LLC, water, environment and public health consultants, and technical editor of Water Technology. He is a former director of both the U.S. EPA’s Drinking Water Standards and the Risk Assessment divisions.