Among wastewaters, spent caustic streams from petroleum refineries and ethylene plants defy standard biological wastewater treatment. That’s why more advanced technologies are used to pretreat them. Today, these industries can add to their tool kits another highly cost-effective pretreatment approach, electro-oxidation, which uses boron doped diamond (BDD) electrodes. Effluent from this process can then be treated by biological wastewater treatment processes. Given the complete oxidation of spent caustic components, it can be safely discharged into the ocean.
Spent caustic refers to expended solutions of water and sodium hydroxide used to “sweeten” hydrocarbon products. They do this by removing “sour” components, such as malodorous and potentially dangerous sulfides and mercaptans, from ethylene, liquefied petroleum gas or other light petroleum products. Extracting these components is important for safety and environmental reasons.
Today, industries can add to their tool kits another highly cost-effective pretreatment approach, electro-oxidation, which uses boron doped diamond (BDD) electrodes.
Production of heavier hydrocarbon products, such as gasoline, diesel and kerosene, can generate spent caustic. Caustic solutions remove corrosive acid oils and other sour components, so these products burn cleaner and are less corrosive to engines or turbines burning them. The acid oils absorbed in the spent caustic from treating heavier products include naphthenic and cresylic acids.
Spent caustic and the need for pretreatment
Regardless of their constituent compounds, spent caustic typically has high chemical oxygen demand (COD) in the range of 20,000 to 300,000 mg/L. For example, sulfidic spent caustic generally has a COD from 10,000 to 60,000 mg/L. Organic spent caustic COD can range from 50,000 to 250,000 mg/L or higher. While spent caustic generated at refineries is generally produced in small quantities (from 0.25 to 10 3/hr), the kilogram COD load is often much higher than the rest of a water treatment facility’s waste streams combined.
Spent caustic contains hazardous, inhibitory and biorefractory chemicals, not to mention high dissolved solids with a salts mass fraction greater than 5 percent by weight. Not only are these compounds hard to treat biologically, but they also can complicate biological treatment processes. Naphthenates, for one, can make biological effluents toxic and have foaming tendencies when agitated or aerated, even in small amounts.
Overview of traditional spent caustic treatment and disposal methods
To understand how electro-oxidation technology advances the evolution of spent caustic treatment, it helps to review traditional methods of its treatment and disposal. These include off-site disposal and on-site treatment options.
Because spent caustic is classified as a hazardous waste, off-site disposal is usually expensive with environmental and legal liabilities. For these reasons, most refineries and ethylene plants choose on-site treatment options, which fall into two categories: physical separation or oxidation processes.
Physical separation
Physical separation involves “acid springing,” which is also referred to as acid-stripping). In this process, the pH of the spent caustic is adjusted to isolate contaminants from their aqueous phase.
Acid springing is among the oldest on-site treatments for spent caustic. By adding enough strong acid, acid oil can separate from the aqueous phase, forming a floating oil layer. It is then skimmed and collected for off-site disposal or for blending into low-grade hydrocarbon products.
Despite removing a significant amount of COD, this approach has drawbacks. At low pH, sulfide and mercaptans tend to be volatile and separate from the aqueous phase. Wet hydrogen sulfide off-gas is extremely corrosive, causing maintenance, odor and health, safety and environmental (HSE) concerns. Such gases must then be disposed of in an acid gas flare or some type of sulfur recovery unit.
In addition, since almost all water-based chemistry is subject to equilibriums, acid springing does not complete the treatment process, but only reduces the amount of sulfidic and organic components in the aqueous brine effluent. Sulfidic, cresylic and naphthenic compounds will still be present in the acidified brine after springing. These constituents remain difficult to degrade biologically and pose significant operating challenges to the wastewater treatment plant (WWTP), including odor and foaming in the aeration basin.
Oxidation
Oxidation converts sulfides to harmless sulfate salts and turns mercaptans into sulfonic acids, neither with odor or HSE issues. The process also converts organic acid molecules into either carbon dioxide and water or organic acids with smaller molecular weights that are easily biodegradable. Oxidation approaches include direct discharge to an on-site WWTP, chemical oxidation, incineration, wet air oxidation, and now electro-oxidation using BDD electrodes (ZEO). While Table 1 compares the results and relative costs from spent caustic on-site treatment technologies, the following briefly explains each one:
- Direct discharge of spent caustic into a biological WWTP requires facilities to commit large throughput capacities to dilution, for even small spent caustic amounts. The WWTP must be closely monitored to control odors, corrosion, foaming, toxicity and oil springing, which often makes this practice unattractive.
- Chemical advanced oxidation is widely used in WWTPs. Although ozone or chlorine can be used as oxidants, the process typically employs hydrogen peroxide catalyzed with ferrous iron. But the latter approach causes a low pH, springing acid oils and liberating sulfides and mercaptans. Of course, the more oxidant used, the better the results. Given the amounts needed, this process can be costly – even for treating small amounts of spent caustic. Plus, safety concerns arise with oxidants potentially mixing with undissolved oil and with storing large amounts of strong oxidizers inside any refinery or ethylene plant.
- Incineration can effectively dispose of spent caustic, but it needs lots of fuel because spent caustic cannot sustain the heat requirements of the incineration process. Also, the incinerator must be specially designed to handle high salt concentrations, and its refractory material will need frequent replacement.
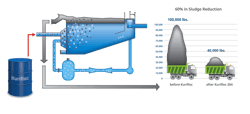
- Wet air oxidation (WAO) applies oxygen from air under heat and pressure to oxidize spent caustic’s aqueous phase. This reduces sulfur species to more benign forms such as sodium sulfate. Complex organic compounds are oxidized to carbon dioxide and biodegradable short-chain organic compounds. After WAO pretreatment, the oxidized spent caustic can be treated in a conventional on-site WWTP. The temperature at which WAO operates determines its oxidation potential – the higher the temperature, the more biodegradable the effluent.
Electro-oxidation using electrically conductive BDD electrodes
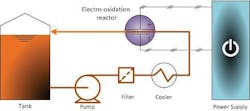
As a commercially available water treatment technology, electro-oxidation has tremendous potential to help refineries manage their spent caustic streams more cost-effectively with fewer safety concerns. Siemens introduced it under the same Zimpro brand name used for its complementary WAO water treatment solutions. Figure 1 shows a simplified diagram of operation.
The Zimpro electro-oxidation process occurs in a reactor containing high-purity, solid polycrystalline BDD electrodes. Using DC power, the electrodes generate highly reactive hydroxyl radicals as the oxidizing agent from the water molecules in the spent caustic aqueous solution. To do this, the electrodes split a hydrogen ion off the H2O molecule.
Electrochemical oxidation electrodes based on BDD technology have been researched for more than 20 years. Diamonds have an inert chemical surface that resists fouling and is not damaged by water’s electrolysis. However, their industrial use in electrochemical advanced oxidation applications has been limited because researchers were unable to develop a commercially feasible electrode able to withstand the harsh environment of spent caustic. However, BDD electrodes with enough oxidation capacity, corrosion resistance and lifetime utility can be cost-effective in large-scale use to treat spent caustic.
Proven results with cost savings and risk reductions
Electrochemical oxidation produces extremely clean effluents. In a study using a pilot system to treat spent caustic of three different compositions, results were as follows:
These results allow for polishing to comply with a WWTP’s end-of-pipe regulatory requirements. Or, in many cases, the process can achieve enough oxidation to allow direct discharge into a sea or ocean. Compared to advanced oxidation processes, no strong oxidizing chemicals must be stored on-site, avoiding the accompanying costs and hazards.
The degree of COD reduction corresponds directly to the electrical current density and amount of time the electro-oxidation system is operated. Sulfidic spent caustic operating expenses – mostly electricity – are stoichiometrically related to the amount of COD destroyed. In fact, with ohmic losses minimized by the high conductivity, the specific energy consumption is approximately 75 kWh per kilogram of COD.
When considering spent caustic treatment options, WAO and electro-oxidation have distinct advantages over other on-site treatment methods. The deciding factor between the two approaches is the amount of spent caustic that needs treatment.
Electro-oxidation technology is ideally suited for treating spent caustic with low flow rates and COD loads. Many refineries can generate inexpensive power on-site, helping lower operational costs much further than oxidation processes dependent on chemical oxidants.
When used for low COD loads, the capital costs of a modular electro-oxidation system are lower than those of typical WAO systems used for larger spent caustic volumes. And it can be expanded as needed or as a site’s demands might dictate.
Mark Clark is a product manager with Siemens Water Solutions. Contact him at [email protected].