Modern electroplating is used in many industries: manufacturing of instruments, electrotechnical production, mechanical engineering, power generation and aerospace applications.
Electrochemical treatment of metal and nonmetal materials offers fine adjustment of structure and thickness of the resulting film, uniform surface treatment in extrusions and in depressions, and production of alloys and composite coats, which could not be obtained with any other technology. These advantages make electroplating usable in nanomaterial production, nanoelectronics, development of new power sources, antibacterial coats and more.
However, electroplating has its drawbacks. Electroplating causes environmental pollution with toxic waste, predominantly heavy metals and their compounds. Such contaminants may be divided into liquid, solid and gaseous.
Liquid waste is wastewater generated by rinsing of parts and equipment, as well as processing and changing of used electrolytes. Solid waste is formed mostly in the process of wastewater or concentrated electrolyte treatment. Gaseous waste includes toxic vapors, aerosols and mixtures of gas and air, emitted during electroplating processes and in waste processing facilities.
This article concentrates on liquid contaminants, i.e., wastewater. Statistically, 30 to 80 percent metal, 5 to 20 percent acid and 2 to 3 percent water are used efficiently in the processes. The most common electroplating processes are zinc, nickel, chrome, copper, cadmium and iron plating, sedimentation of precious metals, and alloys of stannum, lead and bismuth. Part of the metals enter the liquid waste stream. Almost all processes (etching especially) result in large amounts of iron entering wastewater, with mostly steel parts processed. Chrome and cadmium are considered highly hazardous metals (V1). Insufficient wastewater treatment endangers the entire ecosystem: water, ground, plants, animals and humans.
Advantages and drawbacks of electroplating wastewater treatment methods
The main difficulty in electroplating wastewater treatment is the lack of uniformity of composition and concentrations resulting from process type and the amount of water used.
Depending on the chemical composition of waste, there are five types of electroplating wastewater:
Acidic — Generated in the first pickling process, acidic copper, nickel and zinc plating (pH less than 6.5)
Alkaline — Generated in the process of decontamination (pH greater than 8.5)
Contaminated with heavy metal salt — Formed during surface metal treatment and electroplating application (pH less than 6.5)
Cyan-containing — Formed in the process of cyan copper, cadmium and silver plating (pH 2.8 to 11.5)
Chrome-containing — Generated by chrome plating and etching of steel parts (pH 2.3 to 8.8)
The traditional approaches to electroplating wastewater treatment involve chemical reagent, electrochemical and ion exchange methods, as well as adsorption treatment.
The reagent method is one of the simplest and most common methods. In this process, ions of heavy metals are converted into insoluble hydroxides by chemical reactions and subsequent sedimentation and separation. Some of the reagents used are caustic soda, lime and acetylene production byproducts. Special flocculants are used to improve hydroxide coagulation rate and reaction speed.
The reagent method demonstrates low sensitivity to initial contaminant composition, but it has certain drawbacks, namely, high reagent consumption, slow process and, in many cases, its inability to reduce contaminants to maximum contaminant levels (MCL) limitations.
Electrochemical methods of wastewater purification include electrocoagulation, galvanic coagulation, electrochemical destruction and others. These methods are commonly used to remove hexavalent chrome ions from wastewater. First, iron dissolves. Then, the resulting Fe2+ ions reduce hexavalent chrome Cr6+ to trivalent chrome Cr3+ forming hydroxides.
The main difference between electrocoagulation and galvanic coagulation is the way iron is dissolved. The electrocoagulation process involves electrochemical dissociation of iron due to the application of voltage to steel anodes. Galvanic coagulation involves galvanic dissolution of iron due to the difference of potentials on contact between iron and, for example, copper.
Cathode reduction is used to remove ions of lead, stannary, mercury, arsenic and chrome from wastewater. The process converts contaminants into less toxic compounds or substances that are easier to separate from water (gas or solid sediment). The aforementioned metals are sedimented on the cathode and can be recuperated.
In general, electrochemical methods of electroplating wastewater treatment are complicated. Process duration depends on pH of the media, current intensity, wastewater treatment duration and other factors.
Electrochemical methods of electroplating wastewater treatment are complicated. Process duration depends on pH of the media, current intensity, wastewater treatment duration and other factors.
Ion exchange methods of wastewater treatment are based on the ability of cation and anion exchangers to exchange their ions for cations and anions in water solutions and absorb them from the solutions. Ion exchange materials must possess high bulk strength, stability to acids, alkali, oxidants and reduction agents, solvent resistance and minimal volume change. Strong-acid and weak-acid cation exchangers are most commonly used for electroplating wastewater treatment, as well as strong-base and weak-base ion anion exchangers. After saturation, exchangers are regenerated to restore the ion exchange ability. Concentrated salt solutions formed during regeneration can be used in industrial processes or be decontaminated. Their processing is a complex task.
Ion exchange methods reduce the consumption of freshwater in electroplating by 2 to 4 times, while increasing the footprint of treatment facilities by 1.5 to 2 times. This method has other limitations: excessive reagent use (3 to 4 times that of theoretical requirement) to regenerate ion exchangers, high water consumption for ion material washing, and large amounts of mineral salts dumped into water bodies with neutralized regeneration products. The high costs and lack of equipment and ion materials preclude common implementation of ion exchange methods of electroplating wastewater treatment.
Final purification of wastewater to remove heavy metals often involves absorption. This method aims to remove remaining metals that could not be extracted in previous stages with other methods. The most common adsorbent used in wastewater treatment is activated charcoal. This method is limited by sorbent cost, high consumption of reagents for sorbent regeneration and bulky equipment.
Using rotating electromagnetic fields and ferromagnetic particles
None of the previously mentioned methods are free from drawbacks, so it is important to research new ways to increase electroplating wastewater treatment efficiency. Literature mentions positive results of using a rotating electromagnetic field with ferromagnetic particles. This approach can increase efficiency of the reagent method of electroplating wastewater treatment by reducing reagent consumption and reaction times, as well as saving power.
"Literature in electroplating wastewater treatment mentions positive results of using a rotating electromagnetic field with ferromagnetic particles."
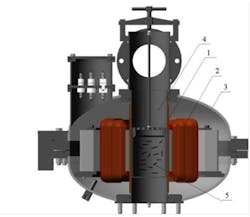
An electromagnetic vortex layer device consists of a chamber inside an inductor coil. The chamber contains ferromagnetic particles. The electromagnetic field causes the particles to rotate and spin, colliding with the walls of the chamber and each other. Reagents in the active zone are intensively mixed. A typical design of such a unit is shown in Figure 1.
Figure 1. A vortex layer unit: 1 – protective collar; 2 – rotating field induction coil; 3 – induction coil case; 4 – nonmagnetic chamber; 5 – ferromagnetic particles. Graphic courtesy of GlobeCore
Constant collisions and friction lead to wear of the ferromagnetic elements and formation of colloidal metal — a good reduction agent. At the same time, electrolysis of water produces hydrogen. Both factors influence the reduction reaction of hexavalent chrome and other heavy metals.
Experiment results and conclusion
For this experiment, wastewater from an electroplating facility was used. Wastewater analysis was performed to define acidity and presence of metals such as iron, copper, nickel, zinc, cadmium and hexavalent chrome. The study of wastewater treatment efficiency was performed in one stage using an AVS-100 unit (see Figure 2) and steel ferromagnetic elements. The results achieved in processing of electroplating wastewater using the AVS-100 are listed in Table 1.
Table 1. Results of electroplating wastewater treatment. Graphic courtesy of GlobeCore
The experiment demonstrated the ability of high-degree purification of wastewater from electroplating production, removing hexavalent chrome, iron, copper, nickel, zinc and cadmium with simultaneous reduction of treatment duration by electromagnetic vortex layer devices with ferromagnetic particles. The processing in the vortex layer only takes several seconds as opposed to 15 to 30 minutes. Reagent consumption was also reduced (100 percent of the theoretical requirement when using the AVS and 150 to 250 percent without the AVS).
Figure 2. AVS-100, an electromagnetic vortex layer device. Graphic courtesy of GlobeCore
Comparison of electric power consumption when using the AVS in the process of reagent treatment compared to using mechanical agitators demonstrates the advantage of the former method: 0.3 kWh per 1 m3 of wastewater, instead of 0.6 to 0.8 kWh.
Wastewater purified with the AVS can be reused in the production process.
Frank May is service manager at GlobeCore in Oldenburg, Germany. With more than 30 years of experience starting, commissioning and operating industrial equipment, he is now focused on purification of domestic and industrial wastewater and regeneration of insulating oils and maintenance of power transformers. May can be reached at [email protected].