Optimizing value for zero liquid discharge by using advanced membrane technology
Increasing water scarcity and tightening restrictions for industrial wastewater discharge are moving more industries to implement zero liquid discharge (ZLD) solutions. While industries such as power have used ZLD technologies for many years as part of plant operations to eliminate wastewater discharge from cooling towers, other industries such as food and beverage manufacturing are now increasing the adoption of ZLD technologies.
To meet local regulations for water management, to reduce environmental impact and to achieve company sustainability goals, ZLD solutions typically require thermal equipment such as falling film evaporators and forced circulation crystallizers to eliminate all plant wastewater. These technologies enable maximum water recovery but require higher-grade alloys to avoid corrosion when the dissolved solids, mainly chlorides, are concentrated beyond their saturation point to crystallization for solids separation from the wastewater brine.
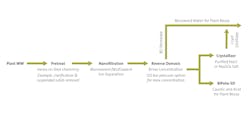
Converting liquid to vapor, and the use of higher-grade alloys makes a thermal ZLD system relatively large and costly. Additionally, more energy is required to sustain evaporation at higher concentration due to the rise of the boiling point when the dissolved solids concentration increases. Therefore, when the goal is to achieve ZLD, the first question to ask is what other technologies can be used to preconcentrate the brine to reduce the required size of the thermal system. Reducing the size of the thermal ZLD system reduces the overall capital cost and energy needed for achieving ZLD.
From the 1980’s to the early 2000’s, thermal evaporators and crystallizers were the primary technologies used to achieve ZLD. Today, and for more than the past decade, membrane technologies such as nanofiltration (NF), reverse osmosis (RO) and electrodialysis (ED) have become common selection options for designing a multi-step ZLD treatment process. Designing an efficient multi-step ZLD solution depends on the effluent profile and other factors. Pretreatment costs, energy costs, chemical costs and membrane replacement costs are important factors when determining how high to push up the brine concentration with membrane technology before transferring the concentrated waste brine to a thermal evaporator or crystallizer for recovering the remaining water.
While there is no “one-size-fits-all” solution, a common approach is to first determine which membrane technology is the best fit for preconcentration for a given application.
Using RO would be a practical option in many cases, however, electrodialysis reversal (EDR) can be a better option than RO for wastewaters with higher organic or silica loads.
For example, a brewery in India used EDR to preconcentrate its waste brine and then used a forced circulation crystallizer to achieve ZLD. The combination of EDR and crystallizer recovered 90% of the wastewater volume as reusable water to reduce overall water usage for the brewery.
EDR is an electrochemical separation process in which ions are transferred through an ion exchange membrane by means of a direct current (DC) potential. EDR has an additional feature of polarity reversal to switch the direction of ion flow, which is a self-cleaning function and a key attribute of the EDR technology.
For the brewery in India, the feed to the EDR had organic concentrations as high as 500 ppm, as measured by the chemical oxygen demand (COD). EDR having a higher tolerance to organics by its means for moving ions via an electric current, and self-cleaning function, was a more suitable option than RO, which would require more extensive pretreatment and chemical cleaning in comparison to the EDR technology.
Extracting value from waste
After deciding what membrane option is most suitable for brine concentration, another important consideration is evaluating the possible economic benefits of recovering minerals from the wastewater stream. This choice not only reduces the amount of waste solids going to landfill, but it can also help offset part of the cost for achieving ZLD by producing a purified commodity salt product that can be sold.
Examples include industrial grade NaCl or Na2SO4, through the use of advance membrane separation technology like nanofiltration (NF), as shown in Figure 1. NF membranes differ functionally from RO by allowing passage of monovalent salts such as NaCl while rejecting divalent ions such as Ca, Mg and SO4.
For example, at a power plant in Zhuozhou City China, the integrated ZLD solution included NF to produce a purified NaCl permeate stream which was then concentrated by RO before final crystallization using thermal technology. For the recovered NaCl product to meet industrial-grade purity requirements, the NF membrane has been designed to provide a large separation factor between monovalent and multivalent ions, achieving greater than 80% separation of calcium and greater than 90% separation of magnesium and sulfates. When NF effectively separates out divalent ions, NF provides another advantage in which it helps minimize required cleaning of the RO membrane when using RO to maximize brine concentration prior to salt crystallization.
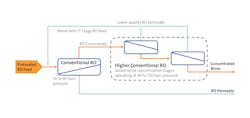
Furthermore, when the brine has been conditioned for mineral recovery, new RO technology can now concentrate brines to 150,000 mg/l NaCl or greater through specially designed membrane elements which can withstand operating pressures of 90-120 bars with minimal compaction. The higher-pressure RO element design combined with use of a more permeable membrane to allow for some of the monovalent ions to pass through to the permeate, maximizes the concentration of the brine stream, to more than double the brine concentration limit of conventional RO operating at 80 bars (Figure 2).
New higher-pressure RO technology reduces energy and cost for recovering minerals from brine by further reducing the required size of the downstream thermal system for crystallization. Higher concentration RO technology can also be applied to mixed-salt brine applications in which the feed chemistry to RO has been properly conditioned to minimize membrane scaling or fouling.
In addition to the option of recovering minerals from industrial waste brine, an emerging solution is being developed to recycle acid and caustic chemical products on-site versus the crystallization of industrial grade NaCl or Na2SO4 products.
For example, the growing battery materials and components manufacturing industry is working with water technology experts to investigate options to achieve ZLD while also reducing waste and chemical usage.
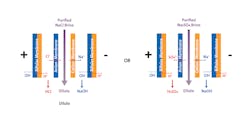
In cases where the manufacturing of components requires acid and caustic chemicals, BiPolar Electrodialysis (BPED) technology has the potential to recover these chemicals from the waste brine for reuse in the component manufacturing process. When NF and other water technologies are used to purify the brine stream, the BPED system can process the purified NaCl or Na2SO4 brine stream as an input along with water, and split ions to recombine them into acid (HCl or H2SO4) and caustic (NaOH) products as outputs. The splitting reaction occurs in the interior of the bipolar ion exchange membranes, and the resulting hydroxide and hydrogen ions combine with the cations and anions from the salt solution respectively (Figure 3).
Conclusion
In conclusion, as the need to achieve ZLD grows, advanced technologies combined with the process expertise for designing multi-step treatment solutions are providing industries with more options to reduce energy and cost for maximizing water reuse and achieving ZLD. When evaluating potential solutions to achieve ZLD, advancements in membrane technology can also reduce the cost for mineral recovery or present the option for acid and caustic recovery, to reduce waste and increase the value of achieving ZLD as an integral part of plant operations and water management strategy.
Josh Dewanaga is a senior product manager for Veolia Water Technologies & Solutions focusing on brine concentration and crystallization applications. During his 15 years in industrial water and wastewater treatment, has was worked on various ZLD projects around the globe and has served as a technical leader for the design of evaporator and crystallizer systems. His experience also extends to leading the development of new brine concentration solutions to best meet the business and environmental needs of customers.