Like the play "Arsenic and Old Lace," arsenic has a complex and dangerous history. It comes in many forms and has the ability to transform between them like a chameleon changing its colors. At the atomic level, arsenic exchanges ions easily, so it can exist in many different chemical forms, and it has the ability to combine readily with other elements. It is this transmutable nature of arsenic that makes it a difficult contaminant to remove from water. It’s almost like playing a game of "Now you see it, now you don’t!"
Arsenic can combine with carbon to form organic arsenic or it can exist as an inorganic compound. The dominant inorganic forms are arsenite, with a valency of 3, and arsenate, with a valency of 5. Valency is a measure of the ability of a compound to combine with other elements. The inorganic forms of arsenic are generally considered to be the most toxic.
Arsenic is even difficult to distinguish by sight because it takes so many different forms. It’s a strange chemical that sometimes acts as a metal and can take the form of a crystalline powder or brittle, silver and metal-like metaloid. Forms of arsenic can be found naturally occurring in rocks and soil around the globe. Concentrations may be higher in certain areas as a result of weathering and man-induced activities such as mining, fossil fuel combustion and pesticide use.
Arsenic is also hazardous? Dangerous hardly scratches the surface of arsenic’s many disguises. It is so toxic that it is one of history’s most notorious poisons. In the Middle Ages, arsenic was used so often as a homicidal weapon within competing royal families that any royal death was suspected to be arsenic poisoning.
Joseph Graziano’s research at Columbia University (2004) has shown that chronic arsenic exposure contributes to reduced cognitive function in children. Long-term exposure to even small amounts of arsenic in drinking water can cause cancer of the skin, lungs, bladder and kidneys. It can also cause skin deformities such as thickening and pigmentation. Arsenic interferes with circulation and normal red blood cell reproduction. In Taiwan, arsenic has been linked to blackfoot disease, where ingesting arsenic through drinking water over time causes decreased circulation in the feet and lower legs, eventually leading to gangrene.
In Bangladesh, extensive tube well installation in the 1970s and 1980s to provide freshwater for rural communities has inadvertently tapped into arsenic-rich groundwater, leading to the mass poisoning of millions of people. The Bangladesh experience serves as a harsh reminder that groundwater sources must always be tested for arsenic.
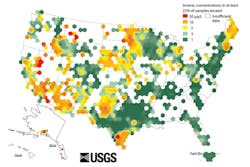
Arsenic is abundant in the U.S. The map in Figure 1 indicates areas of arsenic water contamination across the country. Texas, the Upper Midwest, New England and California are areas of high arsenic concern, as are Oklahoma City, Oklahoma; and Phoenix and Tucson, Arizona. The U.S. Environmental Protection Agency (EPA) has set the acceptable or maximum contaminant level (MCL) of arsenic in water at 10 parts per billion (ppb), but even 10 ppb may be too much. The EPA is currently reviewing that standard, considering that even that low levels of exposure pose considerable risks to humans. It is estimated that as many as 25 million Americans, many of them private water well owners, drink water from a source above the current EPA limit for arsenic.
What can be done to remove arsenic from drinking water?
Because arsenic is so complex, removing it from water is a difficult process. Three water treatment systems noted for arsenic removal: reverse osmosis (RO), adsorption media and distillation. RO systems and adsorption media are only partially effective at arsenic removal while distillation — although sometimes overlooked — is clearly superior at arsenic removal from water.
Several forms of arsenic can be removed to some degree by RO filtration systems, but RO does not remove Arsenic 3 unless it has been pretreated with an oxidizing agent such as chlorine or ozone. In addition to the cost and trouble of pretreatment, RO systems lose effectiveness over time, and with temperature change they require frequent filter membrane replacements and waste copious amounts of rejected water.
Adsorption media water treatment systems offer promise for large-scale arsenic removal. Adsorption media use metal oxides distributed over a flow-bed to form a chemical bond with arsenic in the water. The metal-oxide media are later discarded. In California, the spent media must be disposed of as hazardous waste. Pretreatment is required to remove suspended solids, iron and manganese from the water. Regular media replacement is required because these systems lose efficiency over time.
Distillation as a process for water purification uses a completely different methodology from RO and adsorption media. Instead of removing contaminants from the water as RO and adsorption media attempt to do, distillation removes the water from the contaminants. This is accomplished by first boiling the water into steam, thus breaking the water down into its component parts of H2O and then condensing the steam to pure water in a separate condensing chamber, leaving contaminants behind in the boiling chamber. In the case of arsenic and most other contaminants, distillation results in a 99.9 percent contaminant reduction. In Arizona, where arsenic in groundwater is a major concern, the University of Arizona has called distillation "the most reliable water treatment process for arsenic removal" (The University of Arizona Cooperative Extension publication).
Distillation is often forgotten in the modern water industry discussion as a water treatment process, yet it is superior as a treatment for arsenic and many other contaminants, in particular as a point-of-use (POU) system for drinking water. Opponents of distillation will commonly point to its inability to remove volatile organic gases such as chlorine with a lower boiling point than water. A good distiller will have a venting system and a post filter to address the volatile organic chemical issue. Opponents might also point to the energy input required in the distillation process — 3 kilowatt hours per gallon — as a weakness. At an average cost of 8 cents per kilowatt hour, though, this pure water would cost an average of 24 cents a gallon, which is much lower than bottled water. Critics might also resort to the old "Distilled water is bad for you" or "Distilled water will leach minerals from your body" arguments — both of which are misinformation. Distilled water is simply pure water — nothing more.
Distillation as a process of water treatment and purification does have its limits. It is not compatible with whole-house water treatment because of the energy cost input to distill large quantities of water. But as a POU system for drinking and cooking water, distillation has many technical and scientific advantages for arsenic and other contaminant removal.
If you can’t take the arsenic out of the water, try taking the water out of the arsenic. Try distillation to remove the water from contaminants.
Kyle Foster is manager of business development at Pure & Secure Water LLC, a manufacturer of water purification equipment. He joined the company in January 2015 after evacuating from the Middle East where he worked as a development consultant on water and agriculture projects for 15 years. You can reach him at [email protected].