By Brad Buecker
Cooling towers are a key component at industries around the globe. Proper chemistry control is essential for steady heat transfer in towers and their physical stability as well. But even with diligent chemical feed and monitoring, cooling towers, and especially the tower fill, can accumulate scale and microbiological deposits that inhibit heat exchange and, in worst case scenarios, may induce partial collapse of the tower. This article examines methods to clean tower fill before fouling causes irreversible damage.
Background
Cooling tower performance is highly dependent on the efficiency of contact between the hot return water from heat exchangers and the cool air being pulled or blown through the tower. Heat transfer is enhanced by the use of cooling tower fill, which has evolved into sophisticated designs to maximize air-water contact. An illustration of high efficiency PVC film fill is shown below.
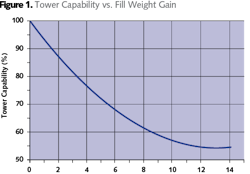
The transition from early splash fill designs to the high efficiency types of today has reduced cooling tower capital and operating costs. However, the generally tortuous path that provides good contact between air and water also makes these fills highly prone to fouling. Advanced fouling results in an approximate 10x weight gain, leading to fill collapse into the sump and expensive fill replacement.Proper Chemical Treatment
While this article focuses on methods to clean tower fill that has begun to accumulate deposits, it is important to understand that proper chemical treatment during normal operation is essential to prevent severe or sudden scaling and fouling problems. With regard to scale (and corrosion) control in cooling systems, the four-decade long methodology of phosphate/phosphonate treatment is giving way to polymer-based, non-phosphorus chemistry for two primary reasons. One is that phosphorus discharges to the environment are being increasingly regulated and restricted due to problems with toxic algae blooms that have afflicted numerous bodies of water. Secondly, the new polymer programs are proving to be more effective for scale and corrosion control in cooling systems. 1The most serious issues in cooling systems are usually related to microbiological fouling. Thus, virtually all systems have as primary treatment some form of oxidizing biocide, most commonly bleach but also possibly gaseous chlorine, bleach/sodium bromide, chlorine dioxide, monochloramine, and monobromamine. One problem at many facilities, particularly in the power industry, is that the regulations developed for and by the U.S. EPA allow no more than 0.2 ppm free available chlorine average residual for 2 hours per day as “Best Available Technology.”2 For plants so constrained, treatment is only allowed for less than 9 percent of any day, thus giving microbes a chance to settle and begin forming protective slime layers.
Options for Dealing with Fouled Fill
Many facilities have suffered from fouled cellular plastic fill. Several have opted to replace the fill in kind while others have chosen low-fouling fill designs that generally feature a more vertical flow pattern, less surface texturing, and sometimes wider spacing between the plates, all at the expense of some cooling efficiency. Since fill replacement can be expensive in terms of both materials and outage time, others have chosen to clean the fill chemically or sometimes mechanically.The choice of replacement vs. cleaning, as well as the cleaning methodology, requires careful consideration. The decision depends on the extent of the fouling, the physical and chemical nature of the foulant, the type of fill, and environmental considerations in dealing with cooling tower blowdown. For example, in heavily fouled film packs, some passages may be completely blocked, preventing the cleaning solution from flowing through and perhaps acting as a filter for solids removed in other parts of the pack. The total mass of deposits if released at once into the recirculating water flow will result in very high suspended solids, and blowdown may have to be diverted or treated prior to discharge.
The type of foulant also varies considerably depending on the nature of the circulating water and the treatment chemistry employed. Over time, the fouling matrix behaves as a filter media, trapping additional suspended solids in the crevices of the fill pack and impeding air and water flow. At this point, the efficiency criteria that constituted the driving force for selecting the fill has become null.
Over time, the high efficiency fill becomes increasingly less efficient, gains as much as 10 times its initial weight, begins to extrude around the supporting beams, and ultimately collapses into the sump. At the point where performance loss becomes very obvious to operators or the fill begins to deform, it is too late to consider cleaning as an option; fill replacement is required. However, if the fouling is detected in its early and moderate stages, several cleaning options are available, depending on the nature of the foulant.Cleaning Options for Cellular Plastic Fill
The most appropriate method for cleaning tower fill depends on several factors, including safety concerns, system metallurgy, in-service vs. out-of-service cleaning, potential impact on plant operations, disposal options for the cleaning solution, impact on the environment, and the chemical and physical nature of the foulant.
Mineral Scales
Hard mineral deposits most commonly consist of silica/silicates or calcium carbonate (calcite). Silica solubility is lowest at low temperature, and deposits often occur near the bottom of the counterflow fill pack where the temperature is lowest, the water is most concentrated, and uneven water/air distribution can lead to dry spots or locally concentrated areas. Calcite deposits often occur throughout the fill pack but are generally heaviest toward the bottom. Higher temperature near the top of the fill pack has the lowest calcite solubility and promotes faster deposition kinetics. However, as the water passes through the fill, the minerals are concentrated slightly by evaporation, and the pH will rise slightly as excess CO2 is stripped.
One technique that can be used effectively on either type of hard scale in its early stages is to apply certain types of surfactants that penetrate the hard deposit and induce it to spall from the slightly flexible plastic substrate. The surfactant is typically applied in addition to the normal scale inhibitor program for an extended period of 60-180 days. This program is never 100 percent effective, but will often result in removal of 70-80 percent of the fouling minerals. Prior to implementing the cleaning process, it is imperative to identify and correct the scaling condition.For large cooling systems where the predominant scale deposit is calcite, the fill can be cleaned by reducing the operating pH and/or cycles of concentration until the water is undersaturated with respect to calcite at the fill conditions. Calcite often serves as the binder for the deposit matrix, so dissolving the calcium carbonate in the deposit matrix can be disproportionately effective.
In principle, any degree of undersaturation will be effective over time. Sulfuric acid is an obvious choice for many plants that already use it for pH control. Other plants may prefer to use safer, less corrosive acids such as organic acids or inhibited sulfamic acid.4 It has been observed that some organic acids are more effective than mineral acids at intermediate pH, and synergistic with sulfuric acid. At pH 5, application of the appropriate organic acid will accelerate the rate of calcite dissolution by 10 to 20 times as compared to sulfuric acid alone.
For predominantly light calcium carbonate scaling, off-line foam acid cleaning has been used very successfully, at least on smaller towers. Strong acid foam is applied by skilled specialists from the top of the fill pack. The nature of the foam allows the acid to contact the scale as it slowly passes downward through the fill. The relatively low volume of spent and mostly neutralized foam cleaning solution is either collected in the sump and disposed of or allowed to mix with other circulating water from neighboring tower cells that may be in service, depending on plant safety and environmental requirements.
Mineral scales can also be mechanically cleaned with some success in situ or ex situ. Due to its brittle nature relative to the flexible PVC, the scale can be dislodged with some success by mechanically cleaning the fill pack in situ from below.
Microbiological/Organic Deposit Matrices
Deposits where microbiological growth or organics serve as the binder for the deposit matrix are characterized by a soft, sometimes putty-like consistency. Unlike mineral scales, deposits of microbiological origin tend to accumulate primarily in the middle of the fill pack. Water velocities directly under the spray nozzles are generally high enough to discourage microbiological adhesion. For this reason, microbiologically initiated fouling sometimes goes undetected because it is not visible on inspections from the top looking down beneath the spray headers. As the water velocity slows down several inches into the fill, microorganisms begin to colonize the surface, acting as a filter for suspended solids passing through the fill.
Fouling tends to be more intense in the middle of the fill than at the bottom because suspended solids are filtered out prior to reaching the bottom layer and because the last few inches of fill do not physically support a thick, soft deposit mass. The inability to clearly view microbiological fouling from either top or bottom, combined with the difficulty of inspecting the middle layers of fill, often allows this type of fouling to progress undetected until it has reached an advanced stage.
Plant personnel have attempted to monitor fill fouling during tower operation using sections of fill suspended from load cells or by cutting an access window into the end of the tower casing to allow a middle section to be removed periodically for inspection using a man lift or by suspending a section of fill beneath the main fill pack to allow it to be easily inspected and weighed. All of these methods can work but none have proven to be totally satisfactory.
Several effective methods exist to remove biological-silt matrix deposits from cooling tower fill. Hyperhalogenation is a widely attempted method, but its effectiveness is usually disappointing. Potential corrosion of system components and the need to dechlorinate prior to discharge are important considerations.
Microbiological matrices often have high water content and will shrink and detach from surfaces when thoroughly dried. However, effectively drying out cooling tower fill can prove problematic even with the help of fans and even if the tower is located in a low humidity climate.
Chlorine dioxide has also been used as a cleaner for cooling tower biofilms with some success. However, the most widely practiced and effective cleaning method for deposits with microbiological or organic binders is hydrogen peroxide (H2O2) due to its oxidizing strength and the physical action of the oxygen micro-bubbles produced as it reacts with organic deposits. The positive environmental profile of hydrogen peroxide involving rapid breakdown to water and oxygen and its ease of application are additional factors favoring peroxide as a tower fill cleaner. Typical dosages are in the range of 500-3,000 ppm active H2O2.
As with most cleaning operations, the addition of low levels of surfactants will help loosen deposits. Polymeric dispersants are generally added to assist in keeping the removed solids in suspension until they can be blown down.
Much of the biomass consists of extracellular and intracellular water and organics that will dissolve with peroxide cleaning. A substantial portion of the deposit typically contains mud and silt that will be released into the water. The images below illustrate the appearance of a slime-clay matrix on moderately fouled high-efficiency cooling tower fill before and after cleaning.
In cases where the deposit contains a high percentage of inorganics, the circulating water can be expected to become highly turbid. The potential for high suspended solids in the cooling tower blowdown should be anticipated when cleaning a severely fouled system and taken into account in the job planning scope.
Summary
Every effort should be made to prevent deposition from occurring. A fill type should be specified that is compatible with reasonable expectations for the system, considering influent water quality, microbiological control, presence or absence of pretreatment equipment, and the possibility for external foulants that might enter the tower through airborne contamination or process fluid leaks.
The microbiological and deposit control program should be diligently monitored to ensure that it is within expectations and delivering the required results. The performance of any pretreatment and sidestream solids removal equipment should be reviewed to ensure such equipment is delivering and maintaining suspended solids within specifications.
Be proactive with inspection and monitoring. There are more options, and less expensive ones, if the fouling is detected at an early stage. Periodic light preventative maintenance tower fill cleanings should be considered. Most high-efficiency fills tend to gain weight slowly over time. Annual preventative maintenance cleanings can stabilize or reverse that trend. If cleaning is indicated, safety and environmental considerations must be adequately addressed.
If fouling occurs, all remediation options should be considered but, generally, the least costly and least aggressive methods applicable to the nature and quantity of the deposit should be the starting point. Identifying and correcting the fouling conditions at an early stage is least expensive and preferable to aggressive cleaning or ultimate fill replacement if the fouling conditions are allowed to persist. IWW
Author’s Note: This article is adapted and condensed from “Effectively Cleaning Cellular Plastic Cooling Tower Fill,” presented by R. Post, K. Emery, G. Dombroski, and M. Fagan at the 33rd Annual Electric Utility Chemistry Workshop, June 11-13, 2013, Champaign, Illinois.
About the Author: Brad Buecker is senior technical publicist with ChemTreat. He has 35 years of experience in or affiliated with the power industry, much of it in steam generation chemistry, water treatment, air quality control, and results engineering. Buecker has a B.S. in chemistry from Iowa State University with additional course work in fluid mechanics, energy and materials balances, and advanced inorganic chemistry. He is a member of the ACS, AIChE, ASME, NACE, the Electric Utility Chemistry Workshop planning committee, and the Power-Gen planning committee.
References
1. Post, R., R. Kalakodimi, and B. Buecker. “An Evolution in Cooling Water Treatment,” PowerPlant Chemistry Journal, 20(6).
2. “Development Document for Effluent Limitation Guidelines and New Source Performance Standards for the Steam Electric Power Point Source Category,” EPA 440/1-74 029-a.
3. Monjoie, Michel, R. Noble, and G. Mirsky. “Research of Fouling Film Fill,” Cooling Technology Institute, TP93-06, New Orleans, La., 1993.
4. Emery, Kevin. “Chemical Cleaning Techniques for Galvanized and Stainless Steel Cooling Towers,” Cooling Technology Institute, Corpus Christi, Texas, 2013.
Circle No. 150 on Reader Service Card