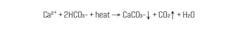A concept often not well recognized is that cooling water chemistry programs are designed in large measure to treat metal surfaces and not just the cooling water itself. For many years over the last century, a common cooling tower treatment program utilized sulfuric acid to minimize scale formation, with chromate chemistry to inhibit corrosion of various metals in the system, most notably carbon steel. Almost universally, this method was discarded in the 1980s due to dawning knowledge of the toxicity of hexavalent chromium (Cr6+).
By far, the most common replacement programs were based on a combination of inorganic and organic phosphate chemistries, with some supplemental chemical additives to control both scaling and corrosion. This treatment method could be quite complex and, for corrosion protection, relied on deposition of reaction products to protect metal surfaces. The deposition products were often subject to process upsets that negatively influenced program effectiveness. More modern methods based on products that directly attach to metal surfaces have now evolved to offer much better metal corrosion protection and to sequester or otherwise inhibit scale formation in cooling waters. This article examines some of these new developments.
Moving Away from the Old Standbys
In the middle of the last century, before the toxicity of hexavalent chromium was recognized, the most common method to protect carbon steel in cooling water systems was based on chromate chemistry for corrosion protection with sulfuric acid feed for scale control. This chemistry is quite straightforward. In most natural waters, and especially when cycled up in a cooling tower, the primary water chemistry issue (apart from microbiological fouling) is calcium carbonate (CaCO3) deposition.
The equation illustrates the inverse solubility of CaCO3 as a function of temperature. Obviously, cooling water temperature rises in heat exchangers, thus making the exchangers susceptible to CaCO3 deposition. The acid-chromate program of old inhibited scaling by reaction of sulfuric acid with bicarbonate ions (HCO3-) to convert the ions to CO2, which escape as gas. A typical pH control range was within or near 6.5 to 7.0. The second compound in the formulation, disodium chromate (Na2Cr2O7), provides chromate ions that react with carbon steel to establish a protective pseudo-stainless-steel layer, which is particularly effective in the oxygen-saturated cooling water generated by cooling towers.
When chromate treatment was discontinued in the 1980s, alternatives obviously became necessary. An early treatment method was feed of phosphates, either orthophosphate (PO4-2) or phosphate complexes that would revert to orthophosphate, to precipitate calcium as Ca3(PO4)2. These programs also provided corrosion protection, as phosphate will react with ferrous ions (Fe+2) produced at anodic sites on carbon steel to form a rate-limiting deposit, while Ca3(PO4)2 precipitates in the local alkaline environment at cathodic sites to inhibit electron transfer. However, even small upsets in phosphate programs can cause severe calcium phosphate fouling, and in fact Ca3(PO4)2 deposition became almost as great a problem as calcium carbonate scaling had been before, a point that was regularly emphasized by the late Paul Puckorius, a noted global cooling water expert. Accordingly, treatment methods evolved to more forgiving methodologies, where in many cases the backbone of these programs are organic phosphates (phosphonates) with a supplemental polymer to sequester and modify the crystal structure of scale-forming ions and compounds (see Fig. 1). Phosphonates attach to deposits as they are forming and disrupt crystal growth and lattice strength. A common treatment program might include one or perhaps two of the organic phosphate compounds in low mg/L dosages for primary scale control, 5–15 mg/L of orthophosphate for additional scale control and corrosion protection, and perhaps 0.5 to 2.5 mg/L of zinc. Zinc reacts with the hydroxyl ions generated at cathodes to form a precipitate [Zn(OH)2], which provides additional cathodic protection. Also typically included in this formulation is 5–10 mg/L of organic polymer for control of calcium phosphate deposition.
Careful monitoring and control of phosphonate-based programs is necessary, as overfeed of phosphonates can lead to calcium-phosphonate deposition. Additionally, phosphonates exhibit varying degrees of susceptibility to degradation from thermal and chemical stresses, where oxidizing biocides in particular may cause component breakdown. Degradation can reduce the effectiveness of the phosphonates, and will also increase the orthophosphate concentration and potential for Ca3(PO4)2 deposition.
Non-Phosphorus Chemistry Evolution
Increasing concern with respect to the impact of phosphorus on the environment, and in particular the growing issue of toxic algae blooms in natural bodies of water, is leading to the evolution of non-phosphorus (non-P) chemistry for cooling water treatment. Also factoring into this evolution is control of metal discharge, including zinc.
Original polymers developed for scale control contained carboxylate functional groups (COO-), where the negatively charged oxygen atoms bind with hardness ions to modify crystal growth. More advanced compounds such as co- and ter-polymers have structures that may include carboxylate, amide (R-CO-NH2), sulfonic acid (SO3H), and other groups for improved reactivity and resistance to degradation. These enhanced polymers have been formulated to help control other deposition, including calcium sulfate, magnesium and calcium silicates, manganese, and calcium fluoride, to name some of the most prominent.
In the absence of any treatment, minerals will deposit as well-ordered crystals on heat exchanger and other cooling system surfaces. Such crystals, whether they be calcium carbonate, one or more of the silicates, or some other species, usually form very tight and tenacious layers on the metal surface.
Polymer scale inhibitors in large measure function by crystal modification, where the chemicals distort and interrupt the normal crystal bonding to produce deposits that remain non-adherent. These loose materials are easily washed away.
Some polymers also act as sequestering agents, in which negatively charged functional groups will bind with cations to prevent them from forming scale. An advantage of polymer chemistry is that each polymer chain can contain many functional groups. Thus, a common range for polymer concentration in the cooling water is 2 to 10 parts per million (ppm).
However, another critical issue relates to the ability of non-P chemistry to inhibit corrosion. Non-P treatments, like phosphate/phosphonate programs, have been designed to operate at an alkaline pH range (7–9), which tends to minimize general corrosion of metals. But even so, corrosion cells can still develop. The key is functionality in which the corrosion inhibitor also establishes a protective barrier on metal surfaces.
One product that has emerged, which goes by the trade name of FlexPro®, combines a group of chemistries that “interact directly with metal surfaces to form a reactive polyhydroxy starch inhibitor (RPSI) complex that is independent of calcium, pH, or other water chemistry constituents.”1 The compounds establish a direct protective layer on metal surfaces, unlike the phosphate/phosphonate programs that rely on deposition of reaction products to form protective barriers, which, as has been noted, can be difficult to control.
Full-scale application of the chemistry has proven very effective. In one instance at a large industrial complex in the southeastern U.S., RPSI replaced previous polyphosphate and then zinc chemistry. Carbon steel corrosion rates have been reduced from 0.2–0.25 mm/yr to 0.0025 to 0.0075 mm/yr. On a secondary note, the change from zinc and then to RPSI was in part influenced by problems with severe algae formation in a clarifier and recycle pond at the plant. The removal of phosphate from the water solved that difficulty.
In another example, the chemistry was applied as a phosphate replacement to both non-contact and direct contact applications at a large steel mill. First, the change eliminated an average of 171,000 pounds of phosphate usage per year. Most importantly, corrosion rates in the non-contact and direct contact applications decreased by 30 percent and 65 percent, respectively.
In the latter case, this can be very important in protecting steel products from corrosion during storage and subsequent transport to finishing plants or other facilities. For the non-contact application at this facility, the chemistry reduced cooling tower blowdown by over one third.
Conclusion
Non-P scale/corrosion control chemistry has been successfully applied at many facilities. However, an important issue with these programs is proper cooling water microbiological control. Microbiological fouling can negatively influence the performance of any scale/corrosion control program. Examples exist where corrosion coupons showed excellent protection but locations within heat exchangers became fouled with microorganisms and suffered flow and heat transfer degradation. Full system analyses are required before application of any program, to be followed by comprehensive monitoring.2 WT
Author’s Note: Results are examples only. They are not guaranteed. Actual results may vary.
References
1. Tribble, R., P. Kalakodimi, R. Post, J. Lamm, and J.L. Nelson. “Advances in Cooling System Passivation and Layup,” presented at the EPRI Cooling Tower Technology Conference, Louisville, Kentucky, August 6, 2015.
2. Post R., B. Buecker, and S. Shulder. “Power Plant Cooling Water Fundamentals,” pre-conference seminar to the 37th Annual Electric Utility Chemistry Workshop, Champaign, Illinois, June 6-8, 2017.