Modern Techniques for Corrosion/Fouling Protection in Mid-Sized Cooling Towers
Much has been written about protection against corrosion, deposition, and microbiological fouling in large power and industrial cooling towers and cooling water systems. However, the hundreds of thousands of moderately sized cooling water systems that supply other industrial and commercial facilities are sometimes overlooked. This article examines several key aspects for optimizing cooling system performance and reliability. Readers can find additional information by investigating the Cooling Technology Institute (www.cti.org), whose annual conference, committee meetings, and library are excellent resources.
Preventing White Rust in Galvanized Cooling Towers
Large cooling towers are often constructed of wood or concrete with plastic fill, fiberglass fan blades and housing, and other non-metallic materials. However, many commercial cooling towers are made of galvanized steel, a strong but low-cost material. For many years, galvanizing has been a well-established technique for protecting steel from the ravages of corrosion, and it is important that new towers be conditioned during initial startup to establish the proper protective coating on the zinc layer for the prevention of white rust corrosion. Towers using water with moderate alkalinity or hardness (more specific guidelines will be offered later in the article) will, for approximately two months after startup, develop a thin, tight and protective layer of hydrated zinc carbonate, whose chemical formula is typically represented as 3Zn(OH)2∙ZnCO3∙H2O. This layer is strongly adherent and nonporous and creates a physical barrier that inhibits corrosion of the underlying zinc base.
However, if conditioning is not performed properly, the protective layer will be loose, allowing for continual zinc corrosion underneath. This product is known as white rust.
“White rust is highly porous and does not provide passivation protection for galvanized metal. Continuous contact of water with the galvanized layer results in continued loss of the zinc layer and eventually exposes the mild steel metal to excessive corrosion and pitting.”1
Several water quality conditions can contribute to white rust formation, including:
• Cooling water pH values above approximately 8.3 or below 6.5
• Calcium concentrations (as CaCO3) below 50 parts-per-million (ppm)
• Total ‘M’ alkalinity (as CaCO3) greater than 300 ppm
• Sulfate (as SO4) greater than 1,200 ppm
• Chloride (as Cl) greater than 450 ppm
Supplemental chemical treatment can be of great benefit in helping form the protective layer and in many applications can be carried over as the primary treatment for subsequent operation. One such technology was outlined in a previous Water Technology article: ChemTreat’s FlexPro cooling water treatment chemistry.2 ChemTreat proprietary FlexPro technology reacts directly with the metal surface and forms a persistent protective barrier on metal surfaces that inhibits corrosion under various operating conditions. If the plant’s discharge permit allows a small concentration of zinc (a 1 ppm limit has been common for years at many facilities), a small amount of zinc additive in the chemical feed solution can enhance passivation. Reference 2 outlines other passivation techniques, but a key point to remember with any program is the need for a pre-operational rinse as stated below.
Thoroughly flush the tower, deck, sides and lines to remove any loose surface dirt and debris. Avoid using acidic or alkaline cleaning aids, as they are known to be corrosive towards galvanized surfaces. Chemical aids can be used to enhance the water cleaning and rinsing process. Alkalinity should be greater than 150 ppm and can be increased using sodium bicarbonate if necessary. Antifoam should be on hand to control excessive foaming.
In all cases, plant personnel should consult the manufacturer’s water chemistry guidelines for both passivation and subsequent routine operation.
Protecting Cooling Tower Fill
Cooling systems provide an ideal warm and wet environment for microbiological growth. Often, water treatment is focused on plant heat exchangers, as microbiological fouling can cause significant heat transfer loss and may lead to underdeposit corrosion of system metals. However, microbiological fouling can also cause problems for cooling tower fill.
Fouling will restrict the flow of air and water through a tower and reduce heat transfer capacity.
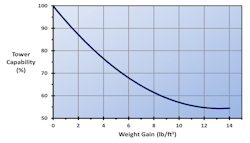
The loss in cooling tower capability as a function of weight gain for a particular type of fill known as the offset flute design is shown in Figure 3.3
Severe fouling, and the subsequent accumulation of weight in the fill, has even been known to cause partial or full tower collapse.
Accordingly, it is quite important to minimize microbial activity throughout the cooling system, including the tower.
Microorganisms are expected to enter a cooling tower through both the makeup water and the air that flows through the tower. Problems arise when the organisms settle on cooling system surfaces and form colonies that generate protective slime layers. The colonies can then continue to grow, while the slime layer gathers suspended solids from the water.
The preferred approach to microbiological control is to kill organisms before they can settle. However, many modern cooling water corrosion and scale control programs establish a pH of approximately 8, which may complicate this approach. The efficacy of chlorine is reduced at even this moderately basic pH, and alternative treatment methods may be necessary. These may include bleach-induced bromine chemistry, monochloramine, and an advanced product from our company, SurfClean™, which provides a controlled chlorine release to optimize contact with microorganisms.
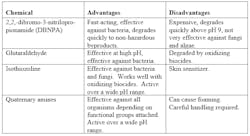
Sometimes, additional treatment may be necessary, and nonoxidizing biocides can be of help. Typically, nonoxidizing biocide feed is performed on a periodic but regular basis, perhaps once per week. Table 1 outlines some of the properties of the most common nonoxidizers.
Careful evaluation of the microbial species in the cooling water is best practice to determine the most effective biocides. Species include bacteria, algae, and fungi, and chemistry that works well for some microorganisms may be less effective against others.
Table 1 – Standard Nonoxidizing Biocides
Work with your internal regulatory team to ensure you have approval to test and use any of these chemicals in accordance with the plant’s National Pollutant Discharge Elimination System (NPDES) permit. Also, as with all chemicals, safety is a critical issue when handling nonoxidizers and following all guidelines on safety data sheets (SDS) is critical for safe handling.
Cleaning Microbiological Deposits from Cooling Towers
There are several effective methods for removing microbiological-silt deposits from cooling tower fill. Hyper-halogenation is one possibility, but the effectiveness may be marginal. Potential corrosion of system components and the need to dechlorinate prior to discharge are important considerations.
Microbiological deposits often have high water content and will shrink and detach from surfaces when dried. However, effectively drying out cooling tower fill can prove problematic even with the help of fans, and even if the tower is located in a low-humidity climate.
Chlorine dioxide has been used as a cleaner for cooling tower biofilms with some success, but the most widely practiced and effective cleaning method for organic deposits employs hydrogen peroxide (H2O2) because of its oxidizing strength and the physical action of the oxygen micro-bubbles produced as the peroxide reacts with organic deposits.4 The positive characteristics of hydrogen peroxide, whose breakdown products are water and oxygen, and its ease of application are additional factors favoring peroxide as a tower fill cleaner. Typical dosages range from 500 to 3,000 ppm active H2O2. As with most cleaning operations, the addition of low levels of surfactants will help loosen deposits. Polymeric dispersants can aid in keeping the removed solids in suspension until they can be blown down.
Figures 4a and 4b illustrate the appearance of slime-silt deposits on moderately fouled high efficiency cooling tower fill before and after cleaning.
In cases where the deposit contains a high percentage of inorganics, the circulating water can become highly turbid. The potential for high suspended solids in the tower blowdown should be anticipated when cleaning a severely fouled system and taken into account in the job planning scope.
Conclusion
As you know, all systems are different. Like all other technologies, due diligence is necessary to determine the feasibility for utilizing methods. Always consult your equipment manuals and guides. ChemTreat personnel have the knowledge and solutions to help you treat your cooling water and steam generation systems
References
- “Pretreatment, Passivation, and Maintenance Recommendations for Galvanized Cooling Towers or Evaporative Condensers”; ChemTreat Technical Bulletin #82, 2021.
- Buecker, B., “Environmental Considerations in the Advancement of Cooling Treatment Technology”; Water Technology, May/June 2021.
- Monjoie, Michel, Noble, Russell, and Mirsky, Gary R., Research of Fouling Film Fill. Cooling Technology Institute, TP93-06, New Orleans, LA, 1993.
- Post, R., Emery, K., Dombrowski, G., and M. Fagan, “Effectively Cleaning Cellular Plastic Cooling Tower Fill”, presented at the 33rd Annual Electric Utility Chemistry Workshop, June 11-13, 2013, Champaign, Illinois.