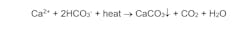As wind and solar renewable energy technologies continue to evolve and mature, fossil-fired power plants, and particularly coal-fired units, will continue to fade away. With the loss of coal-fired plants, there is reduced need in the power industry for makeup water treatment, steam generation chemistry, cooling water treatment and wastewater discharge cleanup expertise.
However, traditional fossil-fueled power plants represent just a fraction of the many industries around the globe that generate steam. Furthermore, other less-carbon-intensive energy production methods continue to evolve, where water/steam chemistry — and cooling water and wastewater treatment — are and will remain critical. These include:
- Cogeneration plants at many heavy industries.
- Combined cycle power plants that will in part, or eventually in whole, operate with hydrogen as the fuel.
- Biomass-fired power plants and biorefineries.
- Small modular nuclear plants.
- Fusion plants sometime in the distant future.
Yet, as many industry experts can attest from experience, often the plant staff focuses on process chemistry and engineering to the detriment of water and steam systems. That is until a failure occurs that shuts down part or all of the plant, or much worse, injures personnel. Some upsets may also place the plant in jeopardy regarding environmental regulations.
This four-part series will examine modern water treatment and chemistry control/monitoring technologies to assist current and future plant employees with complex issues, where even seemingly slight upsets may cause major difficulties. The discussion will frequently have a holistic flavor, as often a treatment method or operational issue is not confined to one system but can influence multiple processes. This first installment will consider makeup water treatment.
A sometimes-overlooked unit operation: Makeup water treatment
Two recent Water Technology articles have examined particular aspects of makeup water treatment1,2. However, neither completely delved into two very important aspects of the process, particularly the second item listed below:
- The choice and complexity of the makeup system is quite variable depending upon the steam generator(s) served. High-pressure units such as those for power production require high-purity makeup, which rely on sophisticated treatment methods. On the other hand, simpler makeup methods are often sufficient for lower-pressure industrial boilers.
- Even though low-pressure boilers can tolerate lower-quality makeup water, industry experts have seen many instances of insufficient makeup system operation and maintenance that allow poor-quality water to enter boilers and cause scaling and corrosion. (Impurities in condensate return can cause similar issues, which will be covered in a later installment of this series.)
The remainder of this article briefly touches on the first item, but primarily focuses on the second.
Makeup water treatment for high-pressure power generators
Impurities in high-pressure boilers can cause deposition and corrosion, and can potentially carry over with steam to induce fouling and corrosion in superheaters, reheaters and turbines. Proper boiler water/steam chemistry control requires high-purity makeup, well-designed condensate/feedwater chemical treatment programs, comprehensive on-line analytical monitoring around the circuit and judicious use of blowdown to maintain boiler water contaminant concentrations within limits, without wasting energy and water. Regarding makeup system effluent purity, Electric Power Research Institute (EPRI) guidelines recommend3:
Guidelines from the International Association for the Properties of Water and Steam (IAPWS) replace sodium with conductivity after cation exchange (CACE) to calculate the influence of carbon dioxide ingress to makeup, which often occurs in vented storage tanks. IAPWS’ recommended CACE limit is 0.1 µS/cm4.
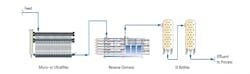
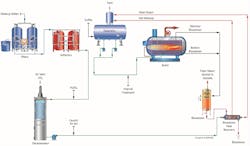
As previously reported in Reference 2, a very common configuration for power plant high-purity makeup is shown in Figure 1.
The micro- or ultrafilter minimizes particulate fouling of the reverse osmosis (RO) membranes. Modern RO units can typically remove over 99% of dissolved ions, leaving a light load for a downstream ion exchange unit or electrodeionization (EDI) system. The ion exchange polisher or EDI unit produces the final, high-purity effluent.
Whereas this configuration or something similar is commonplace for power-generating units, makeup water requirements for industrial boilers are usually less stringent, but still important.
Makeup water treatment for industrial boilers
The American Society of Mechanical Engineers (ASME) recently revised its long-standing guidelines for industrial boiler water purity5. Figure 2 is an extract from that guide.
The key point for this discussion is that lower-pressure boilers can tolerate higher concentrations of impurities because of lower heat fluxes and the reduced potential of solids carryover to steam. (We will examine such data in more detail in a later article in this series on boiler water chemistry, but encourage readers of this article to contact the ASME (www.asme.org) to purchase a copy of the guidelines.)
When it comes to low-pressure boiler water impurities, the largest potential problem is hardness. Note the low feedwater hardness limit of 0.5 mg/L for even the lowest pressure range of Figure 2. For high-pressure units the limit decreases to zero.
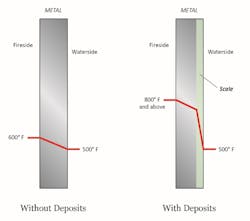
The typical result of hardness excursions is shown below.
A primary reaction is:
Calcium carbonate (CaCO3) scale forms per the inverse solubility of dissolved calcium and bicarbonate alkalinity (HCO3-) with temperature. The general effect of scale deposition on tube wall temperatures is illustrated in Figure 4.
Tube overheating is a common outcome from scale formation. Other deposits may be porous in nature, which can lead to under-deposit corrosion, a topic for future discussion.
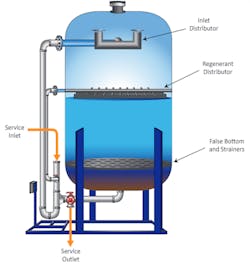
Accordingly, for low-pressure steam generators, a very common core makeup treatment method for years has been sodium softening.
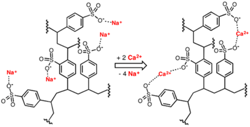
A softener contains many millions of small ion exchange beads and each bead has an enormous number of active sites (typically the sulfonate ion (SO32-)) which, in regenerated form, have sodium ions attached. As water passes through the resin, sodium is exchanged for hardness and other cations that have a stronger affinity for the exchange sites.
A well-designed and operated softener can reduce makeup hardness to very low concentrations, which leads to a key point of this article. From personal experience and via reports seen from colleagues, a common malady at plants with boiler tube failures is frequent malfunction or failure of the sodium softeners. Inspections of extracted failed tubes typically reveal deposits similar to that in Figure 3. Even more disturbing are cases where operators were instructed to bypass the makeup system when the softener malfunctioned and directly feed raw water to the steam generators. Rapid boiler failures were a common outcome, with outage and materials replacement costs reaching six, seven or sometimes even eight figures. Process engineering and chemistry should not supersede water and steam system operation and maintenance. This is a theme that will run through this series.
Softening enhancements and alternatives
Apart from hardness capture, sodium softening alone removes no other ions from the makeup water. In low-pressure boilers with good blowdown control, most impurities may be manageable; however, issues regarding alkalinity deserve additional discussion.
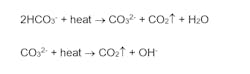
In some corrosion control applications, a mild concentration of HCO3- may be desirable, as the ions can form a mild protective layer on metal surfaces and help reduce the corrosion potential. However, HCO3-, upon reaching the boiler, is in large measure converted to CO2 via the following reactions:
The total conversion of CO2 from the combined reactions may reach 90%. CO2 flashes off with steam and when it re-dissolves in the condensate, CO2 can increase the acidity of condensate return and induce significant carbon steel corrosion.
Accordingly, many softening systems are equipped with downstream equipment to remove alkalinity. A common configuration is shown in Figure 7.
The system includes a forced-draft decarbonator. Acid feed upstream of the tower converts alkalinity to CO2, which escapes in the tower vent. Caustic feed to the decarbonator effluent readjusts the pH. This fundamental arrangement can reduce alkalinity to low part-per-million (ppm) levels. An alternative to the forced-draft decarbonator shown above is a dealkalizer softening arrangement, where a strong base anion (SBA) unit would follow the softeners. SBA resin, when regenerated with brine, puts the resin in the chloride cycle where it can then remove alkalinity.
The system in Figure 7 does nothing to remove other ions including chloride, sulfate and silica. In some cases, it may be beneficial to remove them from the makeup stream, particularly if condensate return to the steam generators is of good quality. In that respect, basic two-stage, single-pass RO is becoming more common as a softener replacement. RO will remove 99% or greater of the total dissolved ions. This allows the boiler cycles of concentration to be raised, resulting in reduced blowdown.
Occasionally, one will see references that call demineralized water “hungry water,” implying that it is similar to acid and will be very aggressive towards metals, particularly carbon steel. As has been demonstrated in the power industry, with proper pH conditioning purified water is not aggressive in that manner.
Conclusion
Inattention to makeup water system operation and maintenance can cause serious deposition and corrosion issues in steam generators. Subsequent failures can be costly and potentially hazardous. This article touched upon several of the most important aspects of makeup water treatment for steam generators.
References
- AlGhamdi, A., “Production of High-Quality Demineralized Water for Steam Generation Units”; Water Technology, July/August 2021.
- Buecker, B. and K. Perryman, “High-purity water treatment: Using membranes to protect membranes”; Water Technology, May/June 2022.
- Comprehensive Cycle Chemistry Guidelines for Combined Cycle/Heat Recovery Steam Generators (HRSGs). EPRI, Palo Alto, CA: 2013. 3002001381.
- International Association for the Properties of Water and Steam, Technical Guidance Document: Volatile treatments for the steam-water circuits of fossil and combined cycle/HRSG power plants (2015).
- Consensus on Operating Practices for the Control of Feedwater and Boiler Water Chemistry in Modern Industrial Boilers, The American Society of Mechanical Engineers, New York, NY, 2021.
Brad Buecker is president of Buecker & Associates, LLC, consulting and technical writing/marketing. Most recently he served as senior technical publicist with ChemTreat, Inc. He has over four decades of experience in or supporting the power industry, much of it in steam generation chemistry, water treatment, air quality control and results engineering positions with City Water, Light & Power (Springfield, Illinois) and Kansas City Power & Light Company’s (now Evergy) La Cygne, Kansas station. Buecker has a B.S. in chemistry from Iowa State University with additional course work in fluid mechanics, energy and materials balances, and advanced inorganic chemistry. He may be reached at [email protected].
Katie Perryman is manager of the pretreatment technical team at ChemTreat. She has nine years in the water treatment industry with a focus on pretreatment applications that include membrane separation and ion exchange systems. She has spent her time at ChemTreat supporting a wide variety of customers in the power, chemical, food and beverage, and transportation industries, among others. Perryman has a B.S. in chemistry from Virginia Tech. She may be reached at [email protected].