In the first two parts of this series, we examined several of the most problematic impurities that can reach steam generators either through poorly operated or maintained makeup water treatment systems, and/or through contaminated condensate return.
These impurities may cause scale formation that leads to tube overheating and under-deposit corrosion (UDC). Some impurities may carry over with steam to cause serious difficulties in downstream equipment including steam superheaters and turbines, which we will examine more closely in later installment.
In this part, we consider treatment programs for large high-pressure drum boilers, virtually all of which — in the United States, at least — were constructed in the last century but are steadily being retired. These programs have undergone substantial evolution since being introduced in the 1930s, whose discussion provides a foundation for later series installments, including Part 4 that will focus on treatment for the heat recovery steam generators (HRSGs) of combined cycle units.
Early boiler water treatment methods
In the early days of power generation, a treatment program might consist of operators putting potato peels or sawdust in the boilers. The tannins and lignin in these natural materials would sequester hardness ions and inhibit them from forming scale compounds such as calcium carbonate or magnesium silicates. Obviously, such treatment methods did not represent rigorous science.
The development of makeup sodium softening as discussed in Part 1 of this series helped ease issues with hardness, but as boiler technology evolved, with increasing pressures and temperatures, internal treatment became much more important. A critical issue, both then and now, is to maintain an alkaline boiler water pH. Iron is an amphoteric metal, meaning that corrosion rates increase at both low and high pH.
Tri-sodium phosphate (Na3PO4) boiler water treatment represented a major step forward to generate alkaline conditions and minimize general corrosion.
Na3PO4 + H2O ⇌ Na2HPO4 + NaOH
We will return to this chemistry shortly. The second major function of phosphate was (and still is in some situations) to mitigate the effects of contaminant in-leakage, and especially hardness. Phosphate reacts directly with calcium to produce calcium hydroxyapatite:
10Ca+2 + 6PO4-3 + 2OH- → 3Ca3(PO4)2•Ca(OH)2↓
Magnesium and silica react with the hydroxide alkalinity produced by phosphate to form the non-adherent sludge, serpentine:
3Mg+2 + 2SiO3- + 2OH- → 3MgO•2SiO2•2H2O↓
Calcium hydroxyapatite and serpentine are much easier to remove than the hard scale that would otherwise form. These sludges typically settle in the boiler mud drum or lower headers from which they are extracted by blowdown.
In early, lower-pressure boilers, phosphate concentrations of 20-40 parts-per-million (ppm) were common to combat hardness ingress. However, as higher-pressure boilers evolved, caustic gouging became problematic.
These failures often occur underneath boiler tube deposits — most commonly porous iron oxides that accumulate via transport of corrosion products from the condensate/feedwater system. Water penetrates the deposit through various channels. And as the water approaches the tube surface, temperatures increase. The water then boils off, leaving impurities behind. This phenomenon is known as wick boiling.
Sodium hydroxide is one species that may remain, and concentrations can rise many times over those in the bulk boiler water. The concentrated NaOH attacks the boiler metal and protective oxide layer via the following reactions:
Fe + 2NaOH → Na2FeO2 + H2↑
Fe3O4 + 4NaOH → 2NaFeO2 + Na2FeO2 + 2H2O
We will examine under-deposit corrosion again in a later section, but caustic gouging during the early years of high-pressure boiler water treatment led to a chemistry evolution that has somewhat come full circle.
One of the first modifications that came out of this effort was coordinated phosphate treatment. As is evident from its chemical formula, the molar ratio of sodium to phosphate in tri-sodium phosphate is three to one. In a coordinated phosphate program, enough di-sodium phosphate (Na2HPO4) is added to maintain a Na/PO4 ratio between 2.8 and 2.2 to 1. (Sometimes chemists might have also included a dash of mono-sodium phosphate, NaH2PO4.)
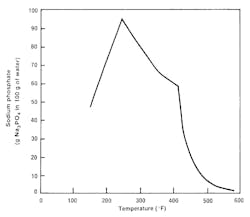
The lower-ratio phosphates shift the equilibrium of Equation 1 to the left, which reduces the concentration of free caustic. Although coordinated treatment was a significant modification to phosphate programs, the evolution did not stop there. Researchers discovered a very important aspect of the sodium phosphates; they are reversely soluble at temperatures above about 250°F, and beyond this temperature will begin to precipitate (hide out) on boiler tube walls.
At the typical temperatures of high-pressure boiler water (600°F or greater), sodium phosphates are only slightly soluble. Chemists found that the precipitate in coordinated programs typically contained a lower sodium-to-phosphate ratio than that in the bulk boiler water, which influenced chemistry. This discovery triggered a modification from coordinated to congruent treatment, per evidence at the time which suggested that the Na/PO4 ratio of phosphate deposits was between 2.3 and 2.6-to-1. This ratio was selected for the new treatment guidelines so that any phosphate which did come out of solution would precipitate “congruently” and not affect chemistry.
However, as analytical chemistry capabilities improved and utility chemists began tracking chemistry ever more closely, data clearly showed that phosphate compounds often precipitated incongruently, with deposit sodium-to-phosphate molar ratios of 2:1 or even lower. Dissolved phosphate concentrations would often drop well below 1 ppm, especially in boilers operating at 2,000 psi and above. Hideout depletes the boiler water of the chemical designed to control scaling and corrosion.
Hideout can be very troublesome in units that cycle up and down in load. Consider a boiler on phosphate treatment at startup. As load is raised and heat fluxes rise, sodium phosphate precipitation increases. Due to incongruent precipitation, the Na/PO4 ratio of the phosphate remaining in the solution rises. This increases the bulk water pH.
The chemist may then add some di-sodium phosphate to elevate the dissolved phosphate concentration and lower the pH, but this phosphate also hides out. When boiler load is reduced, the situation reverses and the precipitated phosphate re-dissolves. In this case, however, the sodium phosphates, which have a low Na/PO4 ratio, drive the pH downward.
Coordinated or congruent phosphate treatment may be impossible to control in boilers that exhibit hideout. Research also indicates that sodium phosphate directly participates in corrosion reactions. The acidity of the low sodium-to-phosphate deposits appears to be a primary factor in this corrosive attack. Acid phosphate corrosion is now suspected as having been a significant culprit in many boiler tube failures that previously were assumed as caustic gouging. Analyses indicate that sodium phosphates form a sodium-iron-phosphate complex with the magnetite layer, as a compound known as maracite.
The development of reliable, high-purity makeup systems (see Part 1 of this series) has greatly minimized the potential for impurity ingress in high-pressure steam generators. So, for these units, the primary goal of boiler water treatment is pH control. The following list highlights important guidelines for phosphate treatment as compiled by a well-known international organization.2
- Tri-sodium phosphate is the only recommended phosphate species. This reduces the potential for acid phosphate deposition. However, the free caustic concentration should be held at or below 1 ppm to minimize the potential for caustic gouging. Computer programs are available, including in spreadsheet format, to optimize boiler water phosphate chemistry and reduce the chances for either acid phosphate or caustic corrosion.
- The recommended TSP concentration range is 0.3 to 2.4 ppm. The low concentration minimizes hideout but still provides some residual in the event of a condenser tube leak or other contaminant ingress. Even this small concentration provides sufficient pH control. Recommended pH ranges vary modestly depending upon boiler design, but typically are within a 9.2 to 9.8 range.
- Because the low phosphate concentration provides limited protection against a condenser tube leak, the unit should be taken offline immediately when an upset is detected, followed by correction of the upset condition.
Difficulties with phosphate hideout led to the development of straight caustic feed as an alternative treatment for high-pressure drum units. Similar to the conditions outlined in the first bullet point, the free sodium hydroxide concentration in caustic treatment should be limited to 1 ppm to protect the boiler internals. Very careful monitoring and control of this chemistry is needed to prevent under-deposit caustic attack. In this era of “lean and mean” plant operation with minimal staff, caustic treatment may be problematic without fully trained personnel to implement and closely monitor the chemistry.
The third bullet point requires additional discussion, which hearkens back to the under-deposit corrosion issue mentioned earlier. Cooling water from a lake or river contains many impurities including suspended and dissolved solids. The latter includes calcium, sodium, magnesium, potassium, bicarbonate, chloride, silica and sulfate, as well as other impurities. These concentrations multiply if the water “cycles up” in a cooling tower. And, of course, seawater-cooled condensers represent another level of potential contamination.
A condenser tube leak introduces these contaminants directly to the high-purity condensate. Numerous reactions are possible if the impurities reach the boiler, as previous equations have suggested. However, the most problematic issue in many cases is represented by the following equation.
MgCl2 + 2H2O → Mg(OH)2↓ + 2HCl
A product of this reaction is hydrochloric acid (HCl). While HCl can cause corrosion in and of itself, the compound will concentrate under deposits where the reaction of the acid with iron generates hydrogen, which in turn can induce hydrogen damage. In this mechanism, atomic hydrogen penetrates into the metal wall and then reacts with carbon atoms in the steel to generate methane (CH4):
4H + Fe3C → 3Fe + CH4
The formation of gaseous methane causes cracking, greatly weakening the steel’s strength and making it susceptible to pressure-induced failures.
Hydrogen damage is troublesome because it cannot be easily detected. After the damage has occurred, the plant staff may replace tubes only to find that other tubes continue to rupture. Hydrogen damage continues to occur at many plants around the world, in large part because plant management does not always recognize the dangers of operation with condenser tube leaks. Also under-recognized is the importance of condensate/feedwater chemistry to minimize the formation of corrosion products that then deposit in boilers and initiate and exacerbate the UDC mechanisms mentioned above.
A note about condensate polishers
This article did not discuss supercritical and ultra-supercritical boilers, where water/steam exist as a single phase. The drum boiler concept, with steam separation in a steam drum, is not viable under supercritical conditions. Very high-purity water is a requirement for supercritical units to prevent contaminants from precipitating on boiler internals. Condensate polishing is an absolute requirement for these units. Polishing is a rare for drum units, as the process adds equipment, maintenance and labor costs for the plant. However, the idea should not be discounted out of hand. A polisher will greatly reduce the potential for contaminant ingress to the boiler, allowing greater flexibility in treatment programs. It may be possible to eliminate the solid alkali treatment programs, phosphate and caustic, and rely on the alkaline conditions generated by ammonia/amine feed to the condensate. Phosphate hideout becomes a moot point in steam generators so equipped.
Conclusion
This installment examined several, but certainly not all, boiler water chemistry issues for conventional high-pressure steam-generating units, many of which have been retired but with quite a few still in operation. Part 4 of this series, which will appear in the December issue, will examine treatment programs for combined cycle HRSGs, which have some similarities to conventional units but, because most HRSGs are of multiple-pressure design, often require significant chemistry adjustments.
References
1. B. Buecker, Tech. Ed., “Water Essentials”; ChemTreat, Inc., 2023. Information is available at www.chemtreat.com.
2. International Association for the Properties of Water and Steam, Technical Guidance Document: Phosphate and NaOH treatments for the steam-water circuits of drum boilers of fossil and combined cycle/HRSG power plants (2015). Available from http://www.iapws.org.
Brad Buecker is president of Buecker & Associates, LLC, consulting and technical writing/marketing. Most recently he served as Senior Technical Publicist with ChemTreat, Inc. He has over four decades of experience in or supporting the power industry, much of it in steam generation chemistry, water treatment, air quality control, and results engineering positions
Buecker & Associates, LLC