The first four parts of this series examined the impurities that can enter steam generators from makeup water and condensate return systems, the problems the contaminants can cause in boilers, and mechanical and chemical control methods. While treatment programs are in large measure designed to protect boilers, also critical is steam chemistry, particularly in systems with turbines. Steam turbines are fine-tuned machines that can be severely damaged, or suffer from aerodynamic efficiency losses, without proper steam chemistry control. We will examine some of the most important issues in that regard in this article.
Steam purity guidelines
Several research organizations have developed steam purity guidelines. Figure 1 is an extract taken from the recent revision of the American Society of Mechanical Engineers (ASME) industrial boiler water guidelines.1 This extract focuses on boiler water and steam impurity level limits for a broad range of industrial boiler pressures. The complete guidelines are available from the ASME at very reasonable cost and should be in the library of any industrial plant with steam generators.
Primary items of importance that will be examined are the decreasing limits for boiler water specific conductance and silica, and the increase in mechanical carryover with boiler pressure. The former is an easily measurable surrogate for the total dissolved solids (TDS) in the boiler water. In the meantime, notice the recommended limit of 20 parts-per-billion (ppb) sodium for all superheated steam conditions. For high-pressure utility units, these recommended concentrations are even lower, as shown in Table 1 per data from the International Association for the Properties of Water and Steam (IAPWS).
Conductivity after cation exchange (CACE) has served for decades as an indirect measurement of chloride (Cl-) and sulfate (SO42-). Online instruments are now available to analyze trace concentrations of these species.3 If chloride and sulfate were included in Table 1, the target value would be the same as sodium, <2 mg/kg. A later section in this article outlines the difficulties these impurities can cause in steam systems.
Impurity pathways to steam
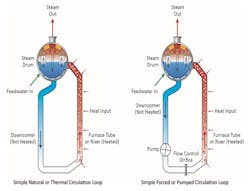
Most industrial steam generators and many (but not all) power boilers are of the drum type. In this design, the water rises through the boiler waterwall tubes and is heated on its way to the drum. Steam flows out of the drum while the remaining water circulates through downcomers to lower headers or drums and then back through the boiler. Condensed steam from the turbine is returned as boiler feedwater, usually through a perforated pipe(s) located in the steam drum.
The three principal impurity ingress pathways to steam are:
- Carryover of moisture droplets from the drum.
- Release of volatile compounds from boiler water. Volatilization typically increases with increasing temperature and pressure.
- Direct introduction via steam attemperating water that comes off the boiler feed pump discharge.
Other factors that can influence steam properties include decomposition of water treatment chemicals such as alkalizing amines, and transport of exfoliated iron oxides from superheaters through the remainder of the steam system.
Mechanical carryover
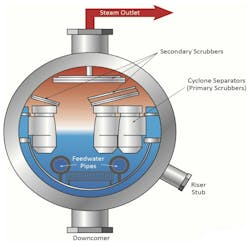
High-purity makeup water is a standard requirement for steam generators with turbines. Even so, impurities will cycle up to some extent in boiler water. The compounds can transport to steam through water droplet carryover. Accordingly, steam drums are typically equipped with separating devices to remove entrained moisture.
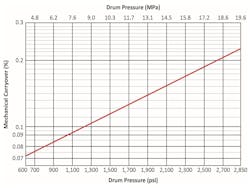
The driving force for separation is the density difference between water and steam. Moisture droplets impinge on separator surfaces and drain back to the drum. However, the density difference narrows with increasing pressure, which reduces separator efficiency. Figure 3 illustrates the increase in mechanical carryover with pressure.
This effect is reflected in the decreasing boiler water specific conductivity limits shown in Figure 1.
Mechanical carryover is exacerbated by other factors including rapid load swings that cause drum surges and boiler water contamination by impurities, e.g., organic compounds, that may generate foam. An example of foam-induced superheater fouling appeared in Part 2 of this series.6
Vaporous carryover
Vaporous carryover is defined as the “fraction of substances entrained from boiler water into the steam by the substance’s volatility, [where] only above a drum pressure of about 2,300 psi does vaporous carryover start to become significant for most of the solids dissolved in boiler water. Below this pressure, it is nearly all mechanical carryover with the exception of a few substances like silica [and] the copper oxides/hydroxides…” 3
Silica does not cause corrosion but will precipitate on turbine blades and reduce aerodynamic efficiency. For older steam generators with copper alloy feedwater heater tubes, feedwater chemistry should be carefully controlled to maintain the copper concentration below 2 ppb.3 Copper is of much less concern nowadays with the phase-out of many coal-fired units in favor of combined cycle and renewable generation. The copper in older units came from corrosion of copper alloy feedwater heater tubes. Personnel at some plants have replaced copper-alloy tubes with ferrous materials including stainless steel.
Steam attemperating water
In virtually all modern steam-generating power units, main- and reheat-steam attemperation water is taken from the discharge of the boiler feed pump. Not only must feedwater be very pure to protect the boiler, but its use for attemperation offers a direct path for contamination of the steam system and turbine. Ammonia, or in some cases an organic amine or ammonia/amine blend, are the only suitable chemicals for feedwater pH control, as solid alkalis such as sodium hydroxide can cause severe and prompt turbine blade and rotor corrosion. For units with steam surface condensers, a condenser tube leak directly contaminates boiler water and steam, apart from any mechanical carryover effects.
Impurity effects in steam systems and turbines
The deposition of impurities is influenced by the behavior of steam as it passes through the turbine. Steam, of course, expands and decreases in pressure as it flows through and transfers energy to the turbine. Silica and copper become less soluble and precipitate on turbine blades. Neither of these compounds is corrosive, but both will reduce the turbine’s aerodynamic efficiency. The loss of even a few megawatts capacity during a peak demand period can be quite costly.
Issues related to offline corrosion in the low-pressure (LP) section of power turbines often represent the most critical issue. Even with units on world-class chemistry programs, trace quantities of salts such as sodium chloride and sodium sulfate carry over into steam. As the steam releases its final superheat energy in the last stages of the LP turbine, moisture droplets begin to form. This region is commonly known as the Phase Transition Zone (PTZ). Salts will concentrate in the moisture and deposit on the blades and rotor. Within normal operation, these salts are not corrosive. However, during unit outages if condenser vacuum is broken and the LP turbine is exposed to ambient air, humidity and oxygen will moisten and activate the salts, which then may initiate pitting on blades, blade attachments and rotors. And this effect is exacerbated if water remains in the condenser hotwell.
With repeated unit cycling, the pits can evolve into microcracks, followed by corrosion fatigue (CF) and stress corrosion cracking (SCC). The potential outcome is blade failure with the turbine in operation, a catastrophic proposition. Nitrogen blanketing has been suggested to protect the LP turbine, but this method has difficulties, including issues related to confined space entry. Less expensive and safer is dry air humidification (DHA) to keep any salts from moisturizing and attacking turbine components.7
For units in which alkalizing amines are employed for feedwater pH control, these organic compounds can decompose in high-temperature superheaters and reheaters to form small-chain organic acids and carbon dioxide. For decades, debate has raged back and forth about the potential effects of the acids on LP turbine components. The corrosion influence appears to be slight, but the carryover of the acids into condensate can depress pH and potentially influence flow-accelerated corrosion (FAC) in the feedwater system.8
Also well-known is mechanical degradation of turbine blades by iron oxide particles that exfoliate from superheater surfaces. This condition has been exacerbated by much more frequent unit cycling due to the need for conventional and combined cycle power plants to follow renewable load production.
Conclusion
A large portion of makeup water preparation and steam generator chemical treatment is designed to minimize impurity carryover to steam. This is especially important for turbine protection. In the next installment, we will summarize the appropriate analytical instrumentation to monitor chemistry throughout the steam-generating circuit.
References
- Consensus on Operating Practices for the Control of Feedwater and Boiler Water Chemistry in Industrial and Institutional Boilers, 2021, The American Society of Mechanical Engineers, New York, NY.
- International Association for the Properties of Water and Steam, Technical Guidance Document: Steam Purity for Turbine Operation (2013). Available from http://www.iapws.org.
- B. Buecker, “Monitoring of Water and Steam Chemistry for Steam Generators”; Chemical Engineering, September 2019.
- B. Buecker (Tech. Ed.), “Water Essentials Handbook”; 2023-2024. ChemTreat, Inc., Glen Allen, VA. Currently being released in digital format at www.chemtreat.com.
- Tomei, G.L., Ed., Steam, its generation and use, 42nd edition, The Babcock & Wilcox Company, Barberton, Ohio, 2015.
- B. Buecker, “The importance of industrial water and steam treatment, part 2”; Water Technology, June 2023.
- Buecker, B., and D. Dixon, “Combined-Cycle HRSG Shutdown, Layup, and Startup Chemistry Control”; Power Engineering, August 2012.
- Shulder, S., and B. Buecker, “Remember the 3Ds of Alkalizing Amines: Dissociation, Distribution, and Decomposition”; PPCHEM Journal, 2023/01.
Brad Buecker is president of Buecker & Associates, LLC, consulting and technical writing/marketing. Most recently he served as Senior Technical Publicist with ChemTreat, Inc. He has over four decades of experience in or supporting the power industry, much of it in steam generation chemistry, water treatment, air quality control and results engineering positions.