Water treatment professionals use chemicals and equipment to control mineral scale, metals corrosion and microbial growth in process applications such as cooling towers. Recent disruptions in raw material supplies have significantly altered how water treatment companies formulate products and, ultimately, service their customers. One primary disruption has been the availability and cost of phosphonate chemistries which are heavily relied upon for the control of mineral scale and mild steel corrosion. The aim of this article is to equip readers with an understanding of the consequences (pros and cons) of using polymers as phosphonate substitutes for mineral scale control.
In order to compare and contrast phosphonates and polymers, this article provides a detailed description of how these additives contribute to the primary mineral scale control functionalities of threshold inhibition, crystal modification, colloidal stabilization and particulate dispersion. Laboratory induction time experiments are used to present HEDP and PBTC phosphonates versus a patented enhanced polymaleic acid (PEPMA) for the control of calcium carbonate. The data presented provide a fair comparison of each technology type along with resulting dosage requirements, limits of use and required formulation adjustments to allow the AWT membership to take an informed approach to substituting polymers for phosphonate technology.
How did we get here?
I started in this industry 36 years ago as an intern in the lab. I think fondly of 1986 as the “good old days” where my worries were making sure my boss would not see any spots on the glassware I had meticulously cleaned and figuring out how I might eke out a C in organic chemistry. My glassware was surely clean. At that time, raw materials were more likely to spawn thoughts of my undercooked hamburger I had impatiently prepared on my small charcoal grill at my apartment than anything to do with polymer or phosphonate supply. How things have changed over the last three-and-a-half decades.
Today, we all find ourselves concerned with supply chain issues. In the water space, we have seen supply chain issues before. I can remember periodic tight supply of acrylic polymers due to plant shutdowns or hurricanes in the gulf coast, molybdate supply constraints and extreme price movements, or the occasional shortage of various specialty ingredients needed to put together water treatment formulations. What I can’t remember is a time during my career where everything has been impacted simultaneously, to the degree we are observing, or for such a long period of time. We are all still struggling to get many raw materials and pricing levels are at an all-time high for most additives utilized in our industry. How did we get here? I am not exactly sure. It seems to all run together between COVID-related issues, labor shortages, government regulations/restrictions, domestic and international transportation bottlenecks, port congestion, energy costs and now war in Europe. It seems that the combination of all these things has led us to this point and created a state of the industry that is not quickly or easily unwound. One type of material that has been most intensely impacted has been organic phosphorous compounds that require the use of yellow phosphorous (P4). This type of phosphorous is necessary for the manufacture of phosphonate starting materials such as PCl3, PCl5 and phosphorous acid. According to www.statista.com1 China is by far the largest producer of phosphate rock in the world with over 50% of global production. In September 2021, the Chinese government published a policy to restrict energy consumption which dramatically impacted the production of yellow phosphorous.2 Since China produces most of the yellow phosphorous and phosphonates such as HEDP and PBTC, this had an immediate impact upon the availability of these molecules and other phosphonate chemistries. Additionally, limited availability of shipping containers, vessels and U.S. port congestion further limited supply and drove up costs. Given these facts, our industry finds itself at a point of limited availability of common phosphonates and pricing that is upward of 2-3 times relative to historical levels.
Why do we need phosphonates?
We have all become used to the functionality and effectiveness of phosphonate compounds in cooling water and other process water applications. For mineral scale control, phosphonates such as HEDP, PBTC and ATMP can be highly effective and used at low concentrations versus polymers and other types of sequestrants and chelates. Some phosphonates can also provide functionality for mild steel corrosion inhibition. These include HEDP, HPA and PSO. We can see the importance of phosphonates by examining a typical cooling water formula. It would be typical to prepare a product that would have some combination of the components listed in Table 1 where one or more components could be a phosphonate.
We can see that phosphonates are important contributors to water treatment formulas. By far, HEDP and PBTC are the most common phosphonates utilized in today’s cooling water treatment applications. ATMP, while highly functional, is used sparingly due to sensitivity to oxidizing biocides such as chlorine and bromine. These phosphonates are predominately utilized for calcium carbonate solubility management and are typically paired with polymers to extend functionality and improve control. As a quick refresher for the common phosphonates employed for scale and corrosion control, we can refer to the following summary:
HEDP — Hydroxyethylidene diphosphonic acid
- Most widely utilized phosphonate in cooling water system
- Effective for calcium carbonate, less effective for sulfate-based mineral scales
- Some efficacy for mild steel corrosion control
- Typical dosage 2-5 ppm active in recirculating water
- Stabilizes CaCO3 (calcite) to about 125-150X saturation3
- Typical use in waters with an LSI of <2.0
- Forms insoluble calcium salts under severe conditions (High Ca2+, high pH)
- Moderately stable to low levels of residual halogen biocide
ATMP — Amino tris (methylenephosphonic) acid
- Once a staple in cooling water systems, now sparingly utilized
- Most efficient phosphonate for calcium carbonate, effective for sulfate-based mineral scales
- Some efficacy for mild steel corrosion control
- Typical dosage 2-3 ppm active in recirculating water
- Stabilizes CaCO3 (Calcite) to about 125-150X saturation3
- Typical use in waters with an LSI of <2.0
- Does not typically form insoluble calcium salts
- Highly sensitive to halogen biocides. Not often utilized with oxidizers. Generates PO4
PBTC — Phosphonobutane tricarboxylic acid
- Widely utilized in cooling water systems
- Increased use in recent years due to improved economics and better functionality versus
- HEDP
- Effective for calcium carbonate, less effective for sulfate-based mineral scales
- Limited efficacy for mild steel corrosion control
- Typical dosage 3-5 ppm active in recirculating water
- Stabilizes CaCO3 (calcite) to about 225X Saturation3
- Typical use in waters with an LSI of >2.0 to ~ 3.0
- Does not typically form insoluble calcium salts under severe conditions
- Highly stable to typical levels of residual halogen biocide
HPA/PSO — Hydroxyphosphono acetic acid (HPA), Phosphinosuccinic Oligomer (PSO)
- Moderate use in cooling water systems
- Primarily function as mild steel corrosion inhibitors
- Secondary efficacy for calcium carbonate
- Typical dosage 5-15 ppm active in recirculating water
- Typical use in waters with an LSI of >1.0 to ~ 3.0
- Requires calcium hardness to function well as a corrosion inhibitor
- Can be paired with zinc to improve performance
- Does not typically form insoluble calcium salts under severe conditions
- HPA highly sensitive to halogen biocides. Generates PO4. PSO, much more stable to typical
- Levels of residual halogen biocide
The advantages of phosphonates are their functionality at low dosages, stability to oxidizing biocides (HEDP, PBTC, PSO), and contribution to mild steel corrosion control (HPA, PSO). Disadvantages include limited functionality. Phosphonates generally are threshold inhibitors but can be poor crystal modifiers and have little to no dispersion properties when compared to polymers. Phosphonates also contain phosphorous which limits or excludes their use in applications requiring low to no phosphorous. However, the primary issues in today’s market are availability and cost of phosphonates. These current drivers highly support the need for finding alternative technologies. One of the primary technologies positioned to replace phosphonate is polymers. More specifically, a newly patented enhanced polymaleic acid (PEPMA) is a great candidate. To examine how PEPMA stacks up, we first need to understand the basics of how these materials function.
Common definitions and mechanisms
In our 2014 report, Ground Up: Designing New Polymers for Independent Water Treatment Companies, we provided an overview of common definitions and mechanisms for scale control. These are particularly useful as we examine how we can evaluate alternate technologies to phosphonates for mineral scale control.
One of the most important concepts to understand when selecting an additive for mineral scale control is Threshold inhibition. Threshold inhibition is the extension of solubility of an otherwise insoluble salt beyond its saturation limits using an additive at sub-stoichiometric levels. This concept of substoichiometric functionality is very important and is what differentiates additives such as polymers and phosphonates from materials that function according to strict stoichiometric ratios such as EDTA. There are a few other key aspects of threshold inhibition that are important to recognize. Generally, threshold inhibition is a temporary effect with respect to time. For example, if uninhibited (untreated) water takes 60 seconds to begin to precipitate calcium carbonate in a given set of conditions (i.e. pH, temperature, calcium concentration and carbonate concentration) and the same water, once treated, extends this time to one hour, then inhibition has occurred with respect to time. The extent and duration of threshold inhibition can be related to a number of factors or conditions. These include, but are not limited to:
- Driving force for precipitation (i.e. pH, temperature, concentration of scale forming ions)
- Particular efficacy of the selected inhibitor
- Other water impurities (both dissolved and suspended)
- Rate of water concentration or evaporation
- Frequency of additive dosage
For calcium carbonate threshold inhibition, phosphonates such as HEDP, PBTC and ATMP are usually more efficient than even the best available polymers. There is a nuance in the commentary here regarding “efficient.” Specifically, phosphonates will typically require a lower dosage to inhibit the formation of calcium carbonate than polymers. However, when used at an effective dose, maleic based polymers such as PEPMA can exceed the control limits (i.e. calcite saturation) when compared to these phosphonates.
Stabilization can be a tricky and controversial topic within the discussion of scale control. Polymers can function as stabilizers whereas phosphonates typically do not provide this functionality. The concept of stabilization can have two meanings with respect to polymer interactions with metal ions: Colloidal stabilization is where precipitation in a fluid (water) occurs, however the polymer or other additive prevents agglomeration of particles beyond 1 micron in size. These particles are thus stabilized via electrostatic interactions with the polymer and remain suspended throughout the water phase. These sub-micron particles are typically not visible to the naked eye. A notable exception to this is stabilized iron particles, which can be visible due to the orange-brown color associated with most oxidized (Fe3+) iron complexes. Colloidal stabilization can fail due to physical or chemical changes in the fluid, which results in particulate agglomeration beyond 1 micron in size and bulk settling of the precipitate. The term stabilization can also be a synonym for sequestration where a coordination complex between the polymer and soluble ions or surface interaction between the polymer and forming crystal lattices occurs and prevention of precipitation is achieved. In this case, threshold inhibition is not the prevailing mechanism since stoichiometry is undefined. Iron stabilization, calcium phosphate stabilization and zinc stabilization are all relevant examples.
Particulate dispersion may be the most straightforward of the concepts for scale control. A formal definition of particulate dispersion is where a mixture of finely divided particles, called the internal phase (often of colloidal size) is distributed in a continuous medium, called the external phase. More simply stated, particulate dispersion is a suspension of particulates in an aqueous solution. These can be inorganic (i.e. calcium carbonate), organic (i.e. biomass, oil, organic debris) or a mixture of the two. Polymer composition and Mw are key determinants in deriving functionality for effective particulate dispersion.
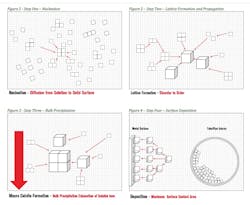
Crystal habit modification is the basis for the control of mineral scales such as calcium carbonate. A crystal habit is defined as the normal size and shape of a precipitated substance in a given set of environmental conditions. The formation of crystals such as calcium carbonate and their subsequent deposition onto surfaces follow a simplified process of nucleation (Figure 1), lattice formation and propagation (Figure 2), bulk precipitation (Figure 3) and surface deposition (Figure 4). Crystal habit modification can be described in instances where a “poison” such as a polymer, phosphonate or other contaminant disrupts normal lattice formation. The crystal lattice poison, in turn, produces crystals that either tend to re-dissolve or precipitate in abnormal forms.
Table 2 provides a simplified overview of the definitions of the primary functionalities of polymers and phosphonates for mineral scale control.
Now that we have reviewed what types of functionalities are important for mineral scale control, we can compare how the patented enhanced polymaleic acid (PEPMA) compares to common phosphonates such as HEDP and PBTC. Table 3 provides an overview comparison. For threshold inhibition, it can be observed that HEDP is effective to a maximum of 150 times calcite saturation whereas PBTC and PEPMA can be effective to 225-250 times calcite saturation.3 From a simple index point of view, this will roughly translate to an Langelier Saturation Index (LSI) limit of about +1.5-2.0 for HEDP and +2.5-3.0 for both PBTC and PEPMA. One important note is that typical dosages for HEDP and PBTC range between 3-5 ppm active in the cooling tower recirculating water where about 8-12 ppm active PEPMA is required in high calcite saturation applications. PEPMA can be applied at lower dosages at lower calcite saturation and where holding time is reduced such as RO and once-through cooling applications.
For calcite crystal modification, HEDP and PBTC do have some effect versus an untreated blank. However, the PEPMA material has best-in-class functionality as a crystal habit modifier. Images 1 and 2 show a comparison between untreated calcite crystals and those treated with PEPMA.
Phosphonates are not particularly good dispersants or suspending aids for mineral scale or other water borne particulates. Best-in-class particulate dispersants are typically polymeric and will have an average molecular weight between ~4,500 Daltons and ~30,000 Daltons. These materials will also tend to have sulfonation and, in many cases, a non-ionic or hydrophobic co-monomer. The catch-22 here is that these types of materials are poor threshold inhibitors and crystal modifiers for calcium carbonate. HEDP and PBTC are discrete, non-polymeric molecules and PEPMA is a very low molecular weight polymer. Table 3 illustrates how the phosphonates have essentially no functionality as particulate dispersants while the PEPMA has limited efficacy due to its low molecular weight. Typically, either phosphonates or PEPMA would be paired with a higher molecular weight copolymer to add particulate dispersion when indicated.
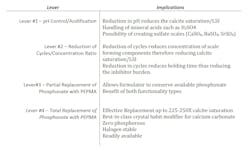
Calcium carbonate control — levers for water treaters
At this point, we have mainly considered an “either/or” decision regarding the use of either phosphonates or polymers for calcium carbonate control. However, there are other options to consider. Table 4 summarizes these options.
Induction time experiments
When discussing threshold inhibition, an example was described where uninhibited (untreated) water takes 60 seconds to begin to precipitate calcium carbonate in a given set of conditions and the same water, once treated, extends this time to one hour. This is an example of induction time or the time in which an additive delays the onset of precipitation. It is important to understand that neither phosphonates nor polymers prevent calcium carbonate formation. Instead, they provide a temporary increase in stability of calcium carbonate. This is typically recognized as an increase in apparent solubility within the observed window of time. This can be demonstrated in one way by looking at additive dosage versus time. For example, we could have the exact same water chemistry and temperature conditions in a once through cooling system and a recirculating cooling system. In the once through system, we may observe that the average time for the water to pass through the condenser is about 10 seconds where in the recirculating system our holding time might be 48 hours. In the once through system, we may require 0.5 ppm active or less of the additive to “inhibit” calcium carbonate where in the recirculating system, we might need 5-10 ppm active to achieve the same results. The only difference here is time. To further make this point, we can look at the situation in reverse. If we were to add 0.5 ppm active additive to our recirculating water, we would likely see precipitation and deposition of calcium carbonate. In this instance, we would consider that the additive was not effective as a calcium carbonate inhibitor. However, this same material is, in fact, a good inhibitor. Our issue in classifying the material is in the lens we used to evaluate dosage versus time. The main point here is that polymers and phosphonates do not infinitely control solubility of scale. They delay the onset of precipitation (induction time). Induction time is impacted by many variables including additive dosage and conditions that impact the driving force for scale precipitation such as temperature, pH, concentration of scale forming ions, co-precipitation, perturbation and surface characteristics. In practical use, we would tend to add enough or excess of the additives in a given combination of conditions and time to ensure we maintained stability for the scale forming species.
There are several ways to compare additives using induction time experiments. For calcium carbonate, we can look for a pH inflection that indicates the onset of precipitation. As calcium carbonate forms in waters that are not highly buffered, we will observe a decrease in pH shortly after the precipitation as we are effectively removing alkalinity from the water via the formation of CaCO3. This technique is one of the simplest ways to screen additives in a lab environment. Another tool that can be used is turbidity. As insoluble calcium carbonate forms, we observe an increase in turbidity which indicates precipitation has occurred. A third method would be to look for mass balance changes in calcium and/or alkalinity via water analysis. This method is least preferred as continuous collection and analysis of water samples is cumbersome and often impractical due to water stability issues or inconsistencies with ion specific electrodes. Lastly, we can also use a tool called a quartz crystal microbalance (QCM). In these instruments, a quartz crystal is set to oscillate at a specific frequency which is measured in Hertz (Hz). The instrument has a quartz crystal resonator that measures the change in frequency as there is deposition of foreign matter onto the crystal. This is an extremely sensitive technique that can measure surface deposition to μg/cm2. Where changes in pH, observation of turbidity or even water chemistry analysis are lagging indicators of scale formation, use of the QCM is an extremely valuable tool for measuring real time changes and observing the onset of scale formation and deposition.
Induction time work presented in this paper includes both experiments using pH inflection as well as QCM deposition measurements.
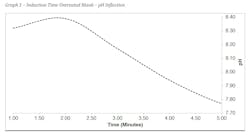
In the pH inflection work, a solution containing 800 ppm calcium (as CaCO3) was heated to 50°C was added to a separate solution containing 800 ppm alkalinity as CO32- which was also heated to 50°C. The resulting water is shown in Table 5.
Graph 1 shows the induction time for an untreated blank sample in these conditions. At about two minutes a pH inflection is observed indicating the precipitation of calcium carbonate. It can also be observed that the desired pH range of 8.8-9.0 was not reached in the untreated blank. This is a good indication that precipitation occurred before the lagging indicator of a pH inflection was measured in the sample.
Graph 2 shows the comparison in the same water conditions of HEDP, PBTC and PEPMA. The phosphonates were treated at 5 ppm active where PEPMA was treated at 10 ppm active. It can be observed that HEDP shows a pH inflection at ~ 350 minutes whereas PBTC and PEPMA do not show failure over the course of the 600-minute (10 hour) evaluation period.
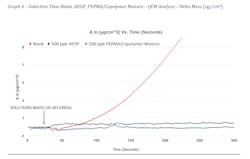
QCM Testing was utilized to compare an untreated blank to HEDP and a mixture of PEPMA and copolymer in a once through utility application. Similar to the pH inflection experiments, a calcium and separate alkalinity solution were prepared and heated to 50°C. These were treated as indicated and mixed starting at 30 seconds. The onset of precipitation of calcium carbonate was measured by frequency changes using the QCM. Table 6 shows the conditions of the evaluation.
Graphs 3 and 4 show the results of the experiment. Changes in frequency can be observed in Graph 3. For the untreated blank, we can see the frequency decrease at about 10-15 seconds after the solutions were mixed. Similarly, HEDP shows an early onset of a small amount of precipitation as indicated by the inflection change at about five seconds after the solutions were mixed. What is different here is that HEDP does not continue to show evidence of precipitation throughout the duration of the test (~150 seconds). The best performer was the PEPMA/Copolymer mixture which shows no evidence of precipitation until about 120 seconds into the test. In this particular application, the additive is only required to control precipitation for 10-20 seconds. Graph 4 shows the mass change or amount of deposition in μg/cm2. We can observe here that the untreated blank shows greater than 9 μg/cm2 deposition of calcium carbonate over the duration of the test while HEDP shows around 0.5 μg/cm2 and the PEPMA/copolymer mixture shows no evidence of deposition.
Conclusions
The water treatment industry has historically relied upon phosphonate technologies for mineral scale and, in some instances mild steel corrosion control. Over the past two-plus years, unreliable supply has necessitated the need for alternatives to phosphonates. This is particularly true for staple products such as HEDP and PBTC. Water treatment professionals have several operational levers such as pH control and limiting cycles to help overcome some of the issues with limited phosphonate supply. Additionally, the functionality of polymers provides a viable alternative due to their efficacy as threshold inhibitors, crystal modifiers, and particulate dispersants. PEPMA has proven to be an effective alternative in laboratory experiments and a history of field use. Water treatment professionals have the option of using this tool as a stand-alone replacement for typical phosphonates such as HEDP and PBTC or as a tool to mitigate their use and stretch supply.
Michael Standish is Vice President – Water Additives at MFG Chemical, LLC. Previously, Mike was founder of Radical Polymers, LLC, a business designed to specifically develop and provide technologies to the independent water treatment community. Mike has 36 years’ experience in water treatment additive design, development and evaluation. Prior to forming Radical Polymers, Mike served as Senior Business Manager for International Specialty Products and Global Business Manager for National Starch's Alco Chemical business. Mike has served on the Board of Directors of AWT and holds a BS in Chemistry and master’s in business administration from the University of Tennessee at Chattanooga.