Strict regulations, rising transportation and treatment costs prompt operators to look at water differently
Oil and gas operators are faced with numerous water challenges — disposal restrictions, water scarcity, and high transportation costs.
Oil and gas operators are faced with numerous water challenges — disposal restrictions, water scarcity, and high transportation costs — making efficient water management strategies in the market in high demand. In fact, an increase in drilling operations across the United States has opened a world of new investment opportunities for both operators and technology companies. At the end of 2017, according to Bluefield Research, “total energy sector spend on U.S. water management [was] forecasted to rise 47 percent” (see “Eight Water Trends to Watch in 2018,” WaterWorld, January 2018).
“The market has gone from a downward cycle to an upswing with the development of different conventional [water] sources,” said Danny Jimenez, CEO of Gradiant Energy Services (Gradiant), a company that specializes in industrial water supply solutions.
Jimenez added that the incredible volume of water required to develop, drill and complete fracking jobs, coupled with disposal challenges, are causing exploration and production (E&P) companies to look at water differently.
“[Water management] is becoming increasingly complex in terms of logistics,” he said. “That translates into cost, and that means that water is the main conversation in every board room and every client and operator has water front and center in their mind, thinking about developing that resource.”
Jimenez added that E&P operators are beginning think about innovative water management as an economic way to enhance their position in the market, and many are looking at water recycling to do so.
“You can go through the logistics of sourcing water from somewhere, trucking or using pipelines to transport the water to your site to do the frack job, and storing the water … or the alternative: to recycle the water coming out of these wells,” he said.
Recently, Gradiant announced a 70,000-barrel-per-day water treatment facility contract with a major operator in the Delaware Basin. The company will employ its reusable clean brine technology, Selective Chemical Extraction (SCE), which takes produced water and purifies it to a level that enables the resulting water to be used again as frack water on-site. The process can reduce sourcing and transportation costs by up to 30 percent, reusing produced water coming from frack wells rather than continually sourcing and transporting it to frack job sites.
Toward 100 percent recovery
Recently, engineers at the University of California, Riverside (UCR) developed a new way to recover almost all the water from highly concentrated salt solutions, which could have implications for traditional hydraulic fracturing waste. The research “will vastly improve the recovery of fresh water during membrane distillation processes,” according to UCR’s Bourns College of Engineering. The process could eliminate the need for disposal in underground injection wells.
The incredible volume of water required to develop, drill and complete fracking jobs, coupled with disposal challenges are causing exploration and production (E&P) companies to look at water differently, according to Gradiant Energy Services.
“In an ideal scenario, thermal desalination would allow the recovery of all the water from brine, leaving behind a tiny amount of a solid, crystalline salt that could be used or disposed of,” David Jassby, who worked on the project, said. “Unfortunately, current membrane distillation processes rely on a constant feed of hot brine over the membrane, which limits water recovery across the membrane to about 6 percent.”
To improve on this, the researchers developed a self-heating carbon-nanotube-based membrane that only heats the brine at the membrane surface. The new system reduced the heat needed in the process and increased the yield of recovered water to close to 100 percent.
From the research: “Controlling the frequency of an applied alternating current at high potentials to a porous thin-film carbon nanotube (CNT)/polymer composite Joule heating element can prevent CNT degradation in ionizable environments such as high-salinity brines.”
The researchers found that “porous CNT/polymer composites can be used as self-heating membranes to directly heat high-salinity brines at the water/vapor interface, achieving high single-pass recoveries that approach 100 percent, far exceeding standard membrane distillation recovery limits.”
Jimenez added that he thinks the industry is moving toward regulations that would require all fracking jobs be done with 100-percent recycled water, as opposed to fresh water transported from another location.
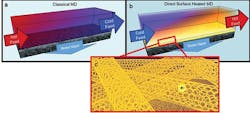
Hot brines used in traditional membrane distillation systems are highly corrosive, making the heat exchangers and other system elements expensive, and limiting water recovery (a). To improve this, UCR researchers developed a self-heating carbon nanotube-based membrane that only heats brine at the membrane surface (b), where the porous carbon nanotube layer acts as a Joule heater (c).
“This notion of ‘100 percent recycling’ is [here], and it is a unique position that we find ourselves in as an industry,” Jimenez said. “It’s right for the environment, and at the same time, it makes perfect economic sense [for the operator].”
Desalination: Down the Line
As the market progresses, desalination technologies could further purify produced water to almost fresh levels. This could open the door for produced water reuse in other industries, including agriculture.
Jimenez said several of his company’s clients overseas, in areas like China, India and the Middle East, have explored desalination of produced water for commercial industry applications. In the U.S., market conditions and current “environmental standards and regulations still have a ways to go to be in agreement in terms of the treatment process,” he said. Still, Gradiant and others are working on improving desalination for this purpose.
A 12-foot-high system in a lab at MIT has demonstrated the principles of the new humidification-dehumidification system, producing about 700 liters of clean water a day. Photo credit: David Castro-Olmedo.
Engineers at MIT, along with the King Fahd University of Petroleum and Minerals in Saudi Arabia, are working on a variation of the standard humidification dehumidification (HDH) process that uses minimal energy to produce clean water from frack water. Originally developed to provide clean drinking water in developing countries, the system treats produced water by “vaporizing water well below the boiling point through direct contact with a carrier gas. The moist air is then bubbled through cooler water, where the purified vapor condenses,” according to MIT.
At MIT, a 12-foot-high test unit ran continuously for weeks, producing about 700 liters of clean water a day. MIT offices say their next step is to scale up to a plant about two to three times the size of this initial unit, which calculations show should be an optimal size. MIT postdoc Prakash Narayan said he expects the first commercial plants could be in operation within about two years.
“Today, the market is mainly a market that is about efficiencies and completing these wells for the least amount of money possible,” Jimenez said. “Thinking about developing water resources in news ways [is one way operators] are handling these issues.” IWW
Alanna Maya is the assistant editor of WaterWorld and Industrial WaterWorld magazines. Email her at [email protected].